Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate renal impairment
- PMID: 24134181
- PMCID: PMC4093924
- DOI: 10.1111/bcp.12260
Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate renal impairment
Abstract
Aims: To evaluate the pharmacokinetics and pharmacodynamics following a single dose of liposomal mifamurtide (L-MTP-PE, MEPACT(®)) in adult subjects with mild (calculated creatinine clearance [CLcr ] of 50-80 ml min(-1)) or moderate (CLcr 30-50 ml min(-1)) renal impairment in comparison with age-, weight- and gender-matched healthy subjects with normal renal function (CLcr >80 ml min(-1)).
Methods: Subjects received a 4 mg dose of liposomal mifamurtide via 1 h intravenous infusion. Blood samples were collected over 72 h for analysis of plasma pharmacokinetics of total and non-liposome-associated (free) mifamurtide and assessment of pharmacodynamics (changes in serum interleukin-6 [IL-6], tumour necrosis factor-α [TNF-α], C-reactive protein [CRP]).
Results: Thirty-three subjects were enrolled: nine with mild renal impairment, eight with moderate renal impairment and 16 healthy subjects. Geometric mean (%CV) AUCinf for total mifamurtide was 89.5 (58.1), 94.8 (27.8), 85.1 (29.0), 95.4 (18.1) nM h in the mild renal impairment, mild-matched healthy subject, moderate renal impairment and moderate-matched healthy subject groups, respectively. Mifamurtide clearance was not correlated with CLcr, estimated glomerular filtration rate or iohexol clearance (all r(2) < 0.01). AUCinf of free mifamurtide was similar across the renal function groups. There were no readily apparent differences in serum pharmacodynamic effect parameters (baseline-adjusted AUEClast for IL-6 and TNF-α and Emax for CRP) between the renal function groups. No subjects reported grade ≥3 or serious adverse events.
Conclusions: Mild or moderate renal impairment does not alter the clinical pharmacokinetics or pharmacodynamics of mifamurtide. No dose modifications appear necessary for these patients based on clinical pharmacologic considerations.
Keywords: MEPACT®; liposomes; mifamurtide; pharmacodynamics; pharmacokinetics; renal impairment.
© 2013 The British Pharmacological Society.
Figures

 ) mild or (
) mild or ( ) moderate renal impairment and (
) moderate renal impairment and ( ) healthy all (normal renal function); and (B) (○) individual, (
) healthy all (normal renal function); and (B) (○) individual, ( ) mean and (
) mean and ( ) geometric mean values of AUCinf by renal function group. (‘healthy all’ = all subjects in the two healthy matched subject groups with normal renal function)
) geometric mean values of AUCinf by renal function group. (‘healthy all’ = all subjects in the two healthy matched subject groups with normal renal function)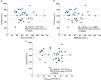
 , individual;
, individual;  , regression;
, regression;  , 95% CI
, 95% CI
 ) mild or (
) mild or ( ) moderate renal impairment and (
) moderate renal impairment and ( ) healthy all (normal renal function); and (B) (○) individual, (
) healthy all (normal renal function); and (B) (○) individual, ( ) mean and (
) mean and ( ) geometric mean values of AUCinf by renal function group. (‘healthy all’ = all subjects in the two healthy matched subject groups)
) geometric mean values of AUCinf by renal function group. (‘healthy all’ = all subjects in the two healthy matched subject groups)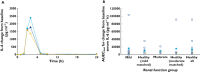
 ) mild or (
) mild or ( ) moderate renal impairment, and (
) moderate renal impairment, and ( ) healthy all (normal renal function); and (B) (○) individual, (
) healthy all (normal renal function); and (B) (○) individual, ( ) mean and (
) mean and ( ) median values of AUEClast by renal function group. (‘healthy all’ = all subjects in the two healthy matched subject groups)
) median values of AUEClast by renal function group. (‘healthy all’ = all subjects in the two healthy matched subject groups)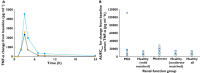
 ) mild or (
) mild or ( ) moderate renal impairment and (
) moderate renal impairment and ( ) healthy all (normal renal function); and (B) (○) individual, (
) healthy all (normal renal function); and (B) (○) individual, ( ) mean and (
) mean and ( ) median values of AUEClast by renal function group. (‘healthy all’ = all subjects in the two healthy matched subject groups)
) median values of AUEClast by renal function group. (‘healthy all’ = all subjects in the two healthy matched subject groups)
 ) mild or (
) mild or ( ) moderate renal impairment and (
) moderate renal impairment and ( ) healthy all (normal renal function); and (B) (○) individual, (
) healthy all (normal renal function); and (B) (○) individual, ( ) mean and (
) mean and ( ) median values of Emax by renal function group. (‘healthy all’ = all subjects in the two healthy matched subject groups)
) median values of Emax by renal function group. (‘healthy all’ = all subjects in the two healthy matched subject groups)Similar articles
-
Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate hepatic impairment.Br J Clin Pharmacol. 2014 Jun;77(6):998-1010. doi: 10.1111/bcp.12261. Br J Clin Pharmacol. 2014. PMID: 24134216 Free PMC article. Clinical Trial.
-
A pharmacokinetic, pharmacodynamic, and electrocardiographic study of liposomal mifamurtide (L-MTP-PE) in healthy adult volunteers.Eur J Clin Pharmacol. 2012 Oct;68(10):1347-55. doi: 10.1007/s00228-012-1262-1. Epub 2012 Mar 30. Eur J Clin Pharmacol. 2012. PMID: 22460239 Clinical Trial.
-
Mifamurtide in metastatic and recurrent osteosarcoma: a patient access study with pharmacokinetic, pharmacodynamic, and safety assessments.Pediatr Blood Cancer. 2014 Feb;61(2):238-44. doi: 10.1002/pbc.24686. Epub 2013 Aug 31. Pediatr Blood Cancer. 2014. PMID: 23997016 Free PMC article.
-
Mifamurtide: a review of its use in the treatment of osteosarcoma.Paediatr Drugs. 2010 Jun;12(3):141-53. doi: 10.2165/11204910-000000000-00000. Paediatr Drugs. 2010. PMID: 20481644 Review.
-
Mifamurtide: CGP 19835, CGP 19835A, L-MTP-PE, liposomal MTP-PE, MLV 19835A, MTP-PE, muramyltripeptide phosphatidylethanolamine.Drugs R D. 2008;9(2):131-5. doi: 10.2165/00126839-200809020-00007. Drugs R D. 2008. PMID: 18298131 Review.
Cited by
-
Mathematical modeling in cancer nanomedicine: a review.Biomed Microdevices. 2019 Apr 4;21(2):40. doi: 10.1007/s10544-019-0380-2. Biomed Microdevices. 2019. PMID: 30949850 Free PMC article. Review.
-
An overview of randomized phase III clinical trials of cancer nanomedicines.Cancer Pathog Ther. 2024 Oct 28;3(4):322-336. doi: 10.1016/j.cpt.2024.10.001. eCollection 2025 Jul. Cancer Pathog Ther. 2024. PMID: 40697515 Free PMC article. Review.
-
Protein nanoparticles in drug delivery: animal protein, plant proteins and protein cages, albumin nanoparticles.J Nanobiotechnology. 2021 May 29;19(1):159. doi: 10.1186/s12951-021-00896-3. J Nanobiotechnology. 2021. PMID: 34051806 Free PMC article. Review.
-
Pivotal Considerations for Optimal Deployment of Healthy Volunteers in Oncology Drug Development.Clin Transl Sci. 2020 Jan;13(1):31-40. doi: 10.1111/cts.12703. Epub 2019 Oct 31. Clin Transl Sci. 2020. PMID: 31674150 Free PMC article. Review.
-
Therapeutic Nanoparticles and Their Targeted Delivery Applications.Molecules. 2020 May 8;25(9):2193. doi: 10.3390/molecules25092193. Molecules. 2020. PMID: 32397080 Free PMC article. Review.
References
-
- Nardin A, Lefebvre ML, Labroquere K, Faure O, Abastado JP. Liposomal muramyl tripeptide phosphatidylethanolamine: targeting and activating macrophages for adjuvant treatment of osteosarcoma. Curr Cancer Drug Targets. 2006;6:123–133. - PubMed
-
- Meyers PA. Muramyl tripeptide (mifamurtide) for the treatment of osteosarcoma. Expert Rev Anticancer Ther. 2009;9:1035–1049. - PubMed
-
- Takeda Pharmaceutical Company Ltd. MEPACT Summary of Product Characteristics. 2012. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info... (last accessed 24 January 2013)
-
- Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M, Kleinerman E, Link MP, Nadel H, Nieder M, Siegal GP, Weiner MA, Wells RJ, Womer RB, Grier HE. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival – a report from the Children's Oncology Group. J Clin Oncol. 2008;26:633–638. - PubMed
-
- Anderson P, Meyers P, Kleinerman E, Oliva C, Liu Y. Mifamurtide (L-MTP-PE) for metastatic and recurrent osteosarcoma (OS): survival and safety profile from a patient access study. Ann Oncol. 2012;23(Suppl. 9):ix488.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

