Nevirapine versus efavirenz-based antiretroviral therapy regimens in antiretroviral-naive patients with HIV and tuberculosis infections in India: a pilot study
- PMID: 24134449
- PMCID: PMC3853651
- DOI: 10.1186/1471-2334-13-482
Nevirapine versus efavirenz-based antiretroviral therapy regimens in antiretroviral-naive patients with HIV and tuberculosis infections in India: a pilot study
Abstract
Background: Administration of rifampicin along with nevirapine reduces the plasma concentration of nevirapine in human immunodeficiency virus positive individuals with concomitant tuberculosis (HIV-TB patients). Nevirapine is a much cheaper drug than its alternative efavirenz, and might be beneficial in resource constrained settings.
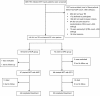
Methods: A randomised open label trial was conducted at All India Institute of Medical Sciences, New Delhi, India. During the regimen of an antiretroviral therapy (ART), naive HIV-TB patients were randomly assigned to receive either nevirapine or efavirenz based ART with concomitant rifampicin based anti-tubercular therapy (ATT). Participants were followed for 24 months after starting ART. The end points were virological, immunological and clinical responses, and progression of HIV disease marked by failure of ART.
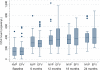
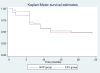
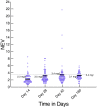
Results: Of the 135 HIV-TB patients, who were receiving rifampicin based ATT, 68 were selected randomly to receive efavirenz based ART and 67 to receive nevirapine based ART. The virological failure rates in the overall population, and the nevirapine and efavirenz groups were 14.1% (19/135); 14.9% (10/67) and 13.2% (9/68), respectively (p =0.94). No significant difference was found between the groups in the rate of clinical, immunological or virological failures. The overall mortality was 17% with no significant difference between the two groups. Except for the lead in period on day 14, the mean nevirapine concentration remained above 3 mg/L. No association was found between plasma levels of nevirapine and incidence of unfavourable outcomes in this group.
Conclusions: Outcome of ART in HIV-TB patients on rifampicin based ATT showed no significant difference, irrespective of whether efavirenz or nevirapine was used. Therefore, nevirapine based ART could be an alternative in the resource limited settings in patients with HIV and tuberculosis co-infection.
Trial registration: NCT No. 01805258.
Trial registration: ClinicalTrials.gov NCT01805258.
Figures

References
-
- Joint United Nations Programme on HIV/AIDS. Global report: UNAIDS report on the global AIDS epidemic. Geneva; 2010. Available: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspubl....
-
- World Health Organization. Global tuberculosis report 2012. Geneva; 2012. Available: http://www.who.int/tb/publications/global_report/en/
-
- National AIDS Control Organisation India, Department of AIDS Control, Ministry of Health & Family Welfare. National AIDS control organization: annual report 2011–12. New Delhi; 2013. Available: http://aidsdatahub.org/en/india-reference-library/item/23889-annual-repo....
-
- World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Geneva; 2010. Available: http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. - PubMed
-
- National AIDS Control Organisation, Ministry of Health & Family Welfare, India. Antiretroviral therapy guidelines for HIV-infected adults and adolescents including post-exposure prophylaxis. New Delhi; 2007. Available: http://www.who.int/bulletin/volumes/88/3/09-068759/en/
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

