Adalimumab maintains remission of Crohn's disease after up to 4 years of treatment: data from CHARM and ADHERE
- PMID: 24134498
- PMCID: PMC4670480
- DOI: 10.1111/apt.12499
Adalimumab maintains remission of Crohn's disease after up to 4 years of treatment: data from CHARM and ADHERE
Abstract
Background: Therapies that maintain remission for patients with Crohn's disease are essential. Stable remission rates have been demonstrated for up to 2 years in adalimumab-treated patients with moderately to severely active Crohn's disease enrolled in the CHARM and ADHERE clinical trials.
Aim: To present the long-term efficacy and safety of adalimumab therapy through 4 years of treatment.
Methods: Remission (CDAI <150), response (CR-100) and corticosteroid-free remission over 4 years, and maintenance of these endpoints beyond 1 year were assessed in CHARM early responders randomised to adalimumab. Corticosteroid-free remission was also assessed in all adalimumab-randomised patients using corticosteroids at baseline. Fistula healing was assessed in adalimumab-randomised patients with fistula at baseline. As observed, last observation carried forward and a hybrid nonresponder imputation analysis for year 4 (hNRI) were used to report efficacy. Adverse events were reported for any patient receiving at least one dose of adalimumab.
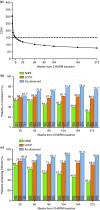
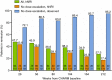
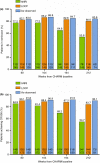
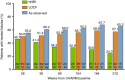
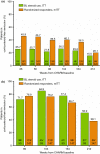
Results: Of 329 early responders randomised to adalimumab induction therapy, at least 30% achieved remission (99/329) or CR-100 (116/329) at year 4 of treatment (hNRI). The majority of patients (54%) with remission at year 1 maintained this endpoint at year 4 (hNRI). At year 4, 16% of patients taking corticosteroids at baseline were in corticosteroid-free remission and 24% of patients with fistulae at baseline had healed fistulae. The incidence rates of adverse events remained stable over time.
Conclusions: Prolonged adalimumab therapy maintained clinical remission and response in patients with moderately to severely active Crohn's disease for up to 4 years. No increased risk of adverse events or new safety signals were identified with long-term maintenance therapy. (clinicaltrials.gov number: NCT00077779).
© 2013 John Wiley & Sons Ltd.
Figures
Comment in
-
Letter: infliximab vs. adalimumab in treating ambulatory perianal fistulising Crohn's disease.Aliment Pharmacol Ther. 2014 Jul;40(2):218-20. doi: 10.1111/apt.12828. Aliment Pharmacol Ther. 2014. PMID: 24946069 No abstract available.
References
-
- Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–66. - PubMed
-
- Carbonnel F, Jantchou P, Monnet E, Cosnes J. Environmental risk factors in Crohn's disease and ulcerative colitis: an update. Gastroenterol Clin Biol. 2009;33(Suppl. 3):S145–57. - PubMed
-
- van Deventer SJ. Review article: targeting TNF alpha as a key cytokine in the inflammatory processes of Crohn's disease–the mechanisms of action of infliximab. Aliment Pharmacol Ther. 1999;13(Suppl. 4):3–8. - PubMed
-
- Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2013 [Epub ahead of print] - PubMed
-
- Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

