A novel and potent inhibitor of dimethylarginine dimethylaminohydrolase: a modulator of cardiovascular nitric oxide
- PMID: 24135074
- PMCID: PMC3868884
- DOI: 10.1124/jpet.113.206847
A novel and potent inhibitor of dimethylarginine dimethylaminohydrolase: a modulator of cardiovascular nitric oxide
Abstract
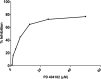
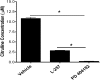
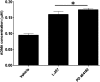
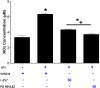
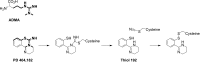
PD 404182 [6H-6-imino-(2,3,4,5-tetrahydropyrimido)[1,2-c]-[1,3]benzothiazine], a heterocyclic iminobenzothiazine derivative, is a member of the Library of Pharmacologically Active Compounds (LOPAC) that is reported to possess antimicrobial and anti-inflammatory properties. In this study, we used biochemical assays to screen LOPAC against human dimethylarginine dimethylaminohydrolase isoform 1 (DDAH1), an enzyme that physiologically metabolizes asymmetric dimethylarginine (ADMA), an endogenous and competitive inhibitor of nitric oxide (NO) synthase. We discovered that PD 404182 directly and dose-dependently inhibits DDAH. Moreover, PD 404182 significantly increased intracellular levels of ADMA in cultured primary human vascular endothelial cells (ECs) and reduced lipopolysaccharide-induced NO production in these cells, suggesting its therapeutic potential in septic shock-induced vascular collapse. In addition, PD 404182 abrogated the formation of tube-like structures by ECs in an in vitro angiogenesis assay, indicating its antiangiogenic potential in diseases characterized by pathologically excessive angiogenesis. Furthermore, we investigated the potential mechanism of inhibition of DDAH by this small molecule and found that PD 404182, which has striking structural similarity to ADMA, could be competed by a DDAH substrate, suggesting that it is a competitive inhibitor. Finally, our enzyme kinetics assay showed time-dependent inhibition, and our inhibitor dilution assay showed that the enzymatic activity of DDAH did not recover significantly after dilution, suggesting that PD 404182 might be a tightly bound, covalent, or an irreversible inhibitor of human DDAH1. This proposal is supported by mass spectrometry studies with PD 404182 and glutathione.
Figures


Similar articles
-
Nitric oxide upregulates dimethylarginine dimethylaminohydrolase-2 via cyclic GMP induction in endothelial cells.Hypertension. 2008 Nov;52(5):903-9. doi: 10.1161/HYPERTENSIONAHA.108.114207. Epub 2008 Sep 29. Hypertension. 2008. PMID: 18824664
-
Overexpression of dimethylarginine dimethylaminohydrolase inhibits asymmetric dimethylarginine-induced endothelial dysfunction in the cerebral circulation.Stroke. 2008 Jan;39(1):180-4. doi: 10.1161/STROKEAHA.107.490631. Epub 2007 Dec 6. Stroke. 2008. PMID: 18063827
-
Estradiol, acting through estrogen receptor alpha, restores dimethylarginine dimethylaminohydrolase activity and nitric oxide production in oxLDL-treated human arterial endothelial cells.Mol Cell Endocrinol. 2013 Jan 5;365(1):11-6. doi: 10.1016/j.mce.2012.08.020. Epub 2012 Sep 7. Mol Cell Endocrinol. 2013. PMID: 22982060
-
Dimethylarginine dimethylaminohydrolase: a new therapeutic target for the modulation of nitric oxide and angiogenesis.Curr Opin Investig Drugs. 2007 Sep;8(9):736-41. Curr Opin Investig Drugs. 2007. PMID: 17729185 Review.
-
The biology and therapeutic potential of the DDAH/ADMA pathway.Curr Pharm Des. 2010;16(37):4089-102. doi: 10.2174/138161210794519246. Curr Pharm Des. 2010. PMID: 21247398 Review.
Cited by
-
High-Throughput Screening for Inhibitors of the SARS-CoV-2 Protease Using a FRET-Biosensor.Molecules. 2020 Oct 13;25(20):4666. doi: 10.3390/molecules25204666. Molecules. 2020. PMID: 33066278 Free PMC article.
-
Inhibitors of the Hydrolytic Enzyme Dimethylarginine Dimethylaminohydrolase (DDAH): Discovery, Synthesis and Development.Molecules. 2016 May 11;21(5):615. doi: 10.3390/molecules21050615. Molecules. 2016. PMID: 27187323 Free PMC article. Review.
-
Metabolite asymmetric dimethylarginine (ADMA) functions as a destabilization enhancer of SOX9 mediated by DDAH1 in osteoarthritis.Sci Adv. 2023 Feb 10;9(6):eade5584. doi: 10.1126/sciadv.ade5584. Epub 2023 Feb 8. Sci Adv. 2023. PMID: 36753544 Free PMC article.
-
Assessment of the direct effects of DDAH I on tumour angiogenesis in vivo.Angiogenesis. 2018 Nov;21(4):737-749. doi: 10.1007/s10456-018-9617-6. Epub 2018 May 2. Angiogenesis. 2018. PMID: 29721731 Free PMC article.
-
The endothelial ADMA/NO pathway in hypoxia-related chronic respiratory diseases.Biomed Res Int. 2014;2014:501612. doi: 10.1155/2014/501612. Epub 2014 Feb 25. Biomed Res Int. 2014. PMID: 24719871 Free PMC article. Review.
References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310 - PubMed
-
- Batra J, Chatterjee R, Ghosh B. (2007) Inducible nitric oxide synthase (iNOS): role in asthma pathogenesis. Indian J Biochem Biophys 44:303–309 - PubMed
-
- Birck M, Holler T, Woodard R. (2000) Identification of a slow tight-binding inhibitor of 3-deoxy-D-manno-octulosonic acid 8-phosphate synthase. J Am Chem Soc 122:9334–9335
-
- Bryan NS, Bian K, Murad F. (2009) Discovery of the nitric oxide signaling pathway and targets for drug development. Front Biosci (Landmark Ed) 14:1–18 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

