Relationship of serum inflammatory biomarkers with plaque inflammation assessed by FDG PET/CT: the dal-PLAQUE study
- PMID: 24135322
- PMCID: PMC3831277
- DOI: 10.1016/j.jcmg.2013.03.009
Relationship of serum inflammatory biomarkers with plaque inflammation assessed by FDG PET/CT: the dal-PLAQUE study
Abstract
Objectives: This study sought to longitudinally investigate the relationship between a broad spectrum of serum inflammatory biomarkers and plaque inflammation assessed by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT).
Background: Both plaque inflammation and serum biomarkers of inflammation are associated with atherothrombotic events; however, the relationship between them is unclear.
Methods: We conducted a post hoc analysis of the dal-PLAQUE (A Randomized Placebo-Controlled Study of the Effect of RO4607381 on Progression or Regression of Atherosclerotic Plaque in Patients With Coronary Heart Disease [CHD] Including Patients With Other CHD Risk Factors), a randomized, placebo-controlled study of dalcetrapib, a cholesteryl ester transfer protein inhibitor, in 130 patients with coronary heart disease, or coronary heart disease risk equivalents on stable lipid-lowering therapy. Baseline and change after 3-month follow-up in inflammatory biomarker levels and baseline and change after 3-month follow-up in aorta and carotid (18)F-FDG PET/CT (mean maximum target-to-background ratio of the most diseased segment [TBRmds]) were analyzed.
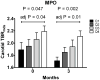
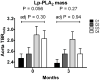
Results: Baseline myeloperoxidase positively correlated with baseline carotid TBRmds (rho = 0.25, p = 0.02). This correlation remained at the 3-month follow-up and was independent of traditional cardiovascular disease risk factors. Baseline lipoprotein-associated phospholipase A2 mass correlated with aorta TBRmds (rho = 0.21, p = 0.03). However, this correlation disappeared at the 3-month follow-up and was not independent of cardiovascular disease risk factors. There was no association between change from baseline in myeloperoxidase or lipoprotein-associated phospholipase A2 mass and change from baseline in aorta and carotid TBRmds. Baseline and change from baseline in high sensitivity C-reactive protein, interleukin 6, soluble P-selectin, soluble E-selectin, soluble intracellular adhesion molecule 1, soluble vascular cell adhesion molecule 1, and matrix-metalloproteinase 3 and 9 did not correlate with baseline or change from baseline in carotid or aorta TBRmds.
Conclusions: Our data show that, in patients with coronary heart disease or at high risk of coronary heart disease on stable lipid-lowering therapy, circulating myeloperoxidase levels are associated with carotid plaque inflammation. (A Randomized, Placebo-controlled Study of the Effect of RO4607381 on Progression or Regression of Atherosclerotic Plaque in Patients With Coronary Heart Disease [CHD] Including Patients With Other CHD Risk Factors [dal-PLAQUE]; NCT00655473).
Keywords: (18)F-FDG PET/CT; IL-6; Lp-PLA(2); MMP; MPO; TBR(mds); atherosclerosis; fluorodeoxyglucose F 18 positron emission tomography; fluorodeoxyglucose F 18 positron emission tomography/computed tomography; high-sensitivity C-reactive protein; hsCRP; inflammatory biomarkers; interleukin 6; lipoprotein-associated phospholipase A(2); matrix metalloproteinase; myeloperoxidase; sE-selectin; sICAM; sP-selectin; sVCAM; soluble E-selectin; soluble P-selectin; soluble intracellular adhesion molecule; soluble vascular cell adhesion molecule; target-to-background ratio of the most diseased segment.
Copyright © 2013 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Disclosures: RD has nothing to disclose. VM receives consulting fees from Tursiop Inc. MW received honoraria from F. Hoffmann-La Roche Ltd. DK and GS are employees of F. Hoffmann-La Roche Ltd. VF has nothing to disclose. JHFR received honoraria from F. Hoffmann-La Roche Ltd. AT received honoraria from F. Hoffmann-La Roche Ltd, Bristol-Myers Squibb and Novartis, and research grants from Merck, Bristol-Myers Squibb, Genentech, GlaxoSmithKline and VBL Therapeutics. MEF received honoraria from F. Hoffmann-La Roche Ltd and acted as a consultant to Genentech. ZAF received research grants from F. Hoffmann-La Roche Ltd, GlaxoSmithKline, Merck, VBL Therapeutics, Novartis, Bristol-Myers Squibb, and Via Pharmaceuticals, and honoraria from F. Hoffmann-La Roche Ltd. F. Hoffmann-La Roche Ltd funded the dal-PLAQUE study and provided third-party editorial support through Prime Healthcare for the preparation of the manuscript.
Figures
References
-
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. - PubMed
-
- van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. - PubMed
-
- Mauriello A, Sangiorgi G, Fratoni S, et al. Diffuse and active inflammation occurs in both vulnerable and stable plaques of the entire coronary tree: a histopathologic study of patients dying of acute myocardial infarction. J Am Coll Cardiol. 2005;45:1585–93. - PubMed
-
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

