Antibody-drug conjugate model fast characterization by LC-MS following IdeS proteolytic digestion
- PMID: 24135617
- PMCID: PMC3929440
- DOI: 10.4161/mabs.26773
Antibody-drug conjugate model fast characterization by LC-MS following IdeS proteolytic digestion
Abstract
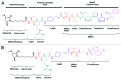
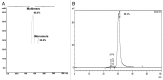
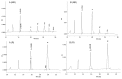
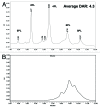
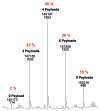
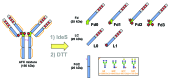
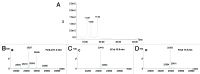
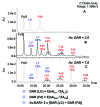
Here we report the design and production of an antibody-fluorophore conjugate (AFC) as a non-toxic model of an antibody-drug conjugate (ADC). This AFC is based on the conjugation of dansyl sulfonamide ethyl amine (DSEA )-linker maleimide on interchain cysteines of trastuzumab used as a reference antibody. The resulting AFC was first characterized by routine analytical methods (SEC, SDS-PAGE, CE-SDS, HIC and native MS), resulting in similar chromatograms,electropherograms and mass spectra to those reported for hinge Cys-linked ADCs. IdeS digestion of the AFC was then performed, followed by reduction and analysis by liquid chromatography coupled to mass spectrometry analysis. Dye loading and distribution on light chain and Fd fragments were calculated, as well as the average dye to antibody ratio (DAR) for both monomeric and multimeric species. In addition, by analyzing the Fc fragment in the same run, full glycoprofiling and demonstration of the absence of additional conjugation was easily achieved. As for naked antibodies and Fc-fusion proteins, IdeS proteolytic digestion may rapidly become a reference analytical method at all stages of ADC discovery, preclinical and clinical development. The method can be routinely used for comparability assays, formulation, process scale-up and transfer, and to define critical quality attributes in a quality-by-design approach.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
