Methods for site-specific drug conjugation to antibodies
- PMID: 24135651
- PMCID: PMC3929454
- DOI: 10.4161/mabs.26632
Methods for site-specific drug conjugation to antibodies
Abstract
Antibody drug conjugates (ADCs) are an emerging class of targeted therapeutics with the potential to improve therapeutic index over traditional chemotherapy. Drugs and linkers have been the current focus of ADC development, in addition to antibody and target selection. Recently, however,the importance of conjugate homogeneity has been realized. The current methods for drug attachment lead to a heterogeneous mixture, and some populations of that mixture have poor in vivo performance. New methods for site-specific drug attachment lead to more homogeneous conjugates and allow control of the site of drug attachment. These subtle improvements can have profound effects on in vivo efficacy and therapeutic index. This review examines current methods for site-specific drug conjugation to antibodies, and compares in vivo results with their non-specifically conjugated counterparts. The apparent improvement in pharmacokinetics and the reduced off target toxicity warrant further development of this site-specific modification approach for future ADC development.
Figures
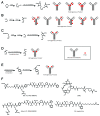
References
-
- Hinman LM, Hamann PR, Wallace R, Menendez AT, Durr FE, Upeslacis J. Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent family of antitumor antibiotics. Cancer Res. 1993;53:3336–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
