Effect of isoniazid therapy for latent TB infection on QuantiFERON-TB gold in-tube responses in adults with positive tuberculin skin test results in a high TB incidence area: a controlled study
- PMID: 24135768
- PMCID: PMC3941252
- DOI: 10.1378/chest.13-1232
Effect of isoniazid therapy for latent TB infection on QuantiFERON-TB gold in-tube responses in adults with positive tuberculin skin test results in a high TB incidence area: a controlled study
Abstract
Background: T-cell interferon-γ release assays (IGRAs) are used in the diagnosis of Mycobacterium tuberculosis infection and could be useful biomarkers of response to treatment of latent TB infection for clinical trials, infection control units, and TB programs.
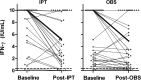
Methods: This investigation was a prospective, controlled substudy of IGRA responses in 82 healthy South African adults with HIV seronegative and positive tuberculin skin test results randomly assigned to treatment with 6 months of daily isoniazid preventive therapy (IPT) or observation before Bacillus Calmette-Guérin revaccination in a clinical trial. QuantiFERON-TB Gold In-Tube (QFT-GIT) assay was used to measure interferon-γ (IFN-γ) response to mycobacterial antigens at baseline and after IPT or observation.
Results: IFN-γ levels declined between baseline and the end of IPT (signed rank test P≤.0001) and between baseline and a similar period of observation without IPT (signed rank test P=.03). The rate of decrease in IFN-γ responses over time did not differ between the groups (Mann-Whitney-Wilcoxon test P=.31). QFT-GIT test results in two subjects (5%) in the IPT group and two subjects (5%) in the observation group reverted from positive to negative during follow-up. No significant difference was found between the groups with respect to baseline positivity or the proportion of patients whose tests reverted to negative.
Conclusions: IPT had no effect on changes in QFT-GIT readouts during short-term follow-up of adults with positive tuberculin skin tests in a high TB incidence setting. QFT-GIT is unlikely to be a useful biomarker of response to treatment of latent TB infection.
Trial registry: ClinicalTrials.gov; No.: NCT01119521; URL: www.clinicaltrials.gov.
Figures
References
-
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC) Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25 - PubMed
-
- National Collaborating Centre for Chronic Conditions; Centre for Clinical Practice at NICE. Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. NICE Clinical Guidelines, No. 117. London, England: National Institute for Health and Clinical Excellence; 2011.
-
- Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, Goletti D. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin Infect Dis. 2004;38(5):754-756 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

