Ultrastructural analysis of nanogold-labeled cell surface microvilli in liquid by atmospheric scanning electron microscopy and their relevance in cell adhesion
- PMID: 24135874
- PMCID: PMC3821644
- DOI: 10.3390/ijms141020809
Ultrastructural analysis of nanogold-labeled cell surface microvilli in liquid by atmospheric scanning electron microscopy and their relevance in cell adhesion
Abstract
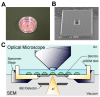
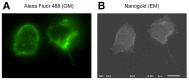
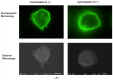
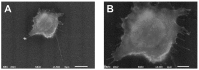
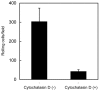
The adhesion of leukocytes circulating in the blood to vascular endothelium is critical for their trafficking in the vasculature, and CD44 is an important cell surface receptor for rolling adhesion. In this study, we demonstrate the correlative observation of CD44 distribution at the lymphocyte cell surface in liquid by fluorescence optical microscopy and immuno-electron microscopy using an atmospheric scanning electron microscope (ASEM). The ultrastructure of the cell surface was clearly imaged by ASEM using positively charged Nanogold particles. ASEM analysis demonstrated microvilli projections around the cell surface and the localization of CD44 on the microvilli. Treatment of cells with cytochalasin D resulted in a loss of the microvilli projections and concomitantly abrogated CD44-mediated adhesion to its ligand hyaluronan. These results suggest the functional relevance of microvilli in CD44-mediated rolling adhesion under shear flow.
Figures

Similar articles
-
The transmembrane domains of L-selectin and CD44 regulate receptor cell surface positioning and leukocyte adhesion under flow.J Biol Chem. 2010 Apr 30;285(18):13490-7. doi: 10.1074/jbc.M110.102640. Epub 2010 Mar 8. J Biol Chem. 2010. PMID: 20212041 Free PMC article.
-
Dual role of melanoma cell adhesion molecule (MCAM)/CD146 in lymphocyte endothelium interaction: MCAM/CD146 promotes rolling via microvilli induction in lymphocyte and is an endothelial adhesion receptor.J Immunol. 2007 Nov 15;179(10):6673-85. doi: 10.4049/jimmunol.179.10.6673. J Immunol. 2007. PMID: 17982057
-
Positively charged nanogold label allows the observation of fine cell filopodia and flagella in solution by atmospheric scanning electron microscopy.Microsc Res Tech. 2014 Feb;77(2):153-60. doi: 10.1002/jemt.22322. Epub 2013 Dec 16. Microsc Res Tech. 2014. PMID: 24343867
-
CD44 and the adhesion of neoplastic cells.Mol Pathol. 1997 Apr;50(2):57-71. doi: 10.1136/mp.50.2.57. Mol Pathol. 1997. PMID: 9231152 Free PMC article. Review.
-
Simulation of cell rolling and adhesion on surfaces in shear flow. Microvilli-coated hard spheres with adhesive springs.Cell Biophys. 1991 Apr;18(2):145-82. doi: 10.1007/BF02989811. Cell Biophys. 1991. PMID: 1726526 Review.
Cited by
-
Obesity Contributes to Ovarian Cancer Metastatic Success through Increased Lipogenesis, Enhanced Vascularity, and Decreased Infiltration of M1 Macrophages.Cancer Res. 2015 Dec 1;75(23):5046-57. doi: 10.1158/0008-5472.CAN-15-0706. Epub 2015 Nov 16. Cancer Res. 2015. PMID: 26573796 Free PMC article.
-
Ca2+-ATPase Molecules as a Calcium-Sensitive Membrane-Endoskeleton of Sarcoplasmic Reticulum.Int J Mol Sci. 2021 Mar 5;22(5):2624. doi: 10.3390/ijms22052624. Int J Mol Sci. 2021. PMID: 33807779 Free PMC article.
-
Cryopreserved mouse fetal liver stromal cells treated with mitomycin C are able to support the growth of human embryonic stem cells.Exp Ther Med. 2014 Sep;8(3):935-942. doi: 10.3892/etm.2014.1801. Epub 2014 Jun 23. Exp Ther Med. 2014. PMID: 25120627 Free PMC article.
-
Direct Evidence of Lack of Colocalisation of Fluorescently Labelled Gold Labels Used in Correlative Light Electron Microscopy.Sci Rep. 2017 Mar 20;7:44666. doi: 10.1038/srep44666. Sci Rep. 2017. PMID: 28317888 Free PMC article.
-
Pyrene Excimer-Based Fluorescent Labeling of Cysteines Brought into Close Proximity by Protein Dynamics: ASEM-Induced Thiol-Ene Click Reaction for High Spatial Resolution CLEM.Int J Mol Sci. 2020 Oct 13;21(20):7550. doi: 10.3390/ijms21207550. Int J Mol Sci. 2020. PMID: 33066147 Free PMC article.
References
-
- Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. - PubMed
-
- Lesley J., Hyman R., Kincade P.W. CD44 and its interaction with extracellular matrix. Adv. Immunol. 1993;54:271–335. - PubMed
-
- Maiti A., Maki G., Johnson P. TNF-α induction of CD44-mediated leukocyte adhesion by sulfation. Science. 1998;282:941–943. - PubMed
-
- Brown K.L., Maiti A., Johnson P. Role of sulfation in CD44-mediated hyaluronan binding induced by inflammatory mediators in human CD14+ peripheral blood monocytes. J. Immunol. 2001;167:5367–5374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

