Ginsenoside Rb1 attenuates oxygen-glucose deprivation-induced apoptosis in SH-SY5Y cells via protection of mitochondria and inhibition of AIF and cytochrome c release
- PMID: 24135936
- PMCID: PMC6270437
- DOI: 10.3390/molecules181012777
Ginsenoside Rb1 attenuates oxygen-glucose deprivation-induced apoptosis in SH-SY5Y cells via protection of mitochondria and inhibition of AIF and cytochrome c release
Abstract
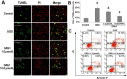
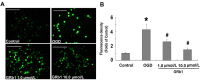
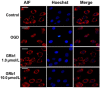
To investigate the role of mitochondria in the protective effects of ginsenoside Rb1 on cellular apoptosis caused by oxygen-glucose deprivation, in this study, MTT assay, TUNEL staining, flow cytometry, immunocytochemistry and western blotting were used to examine the cellular viability, apoptosis, ROS level, mitochondrial membrane potential, and the distribution of apoptosis inducing factor, cytochrome c, Bax and Bcl-2 in nucleus, mitochondria and cytoplasm. We found that pretreatment with GRb1 improved the cellular viability damaged by OGD. Moreover, GRb1 inhibited apoptosis in SH-SY5Y cells induced by OGD. Further studies showed that the elevation of cellular reactive oxygen species levels and the reduction of mitochondrial membrane potential caused by OGD were both counteracted by GRb1. Additionally, GRb1 not only suppressed the translocation of apoptosis inducing factor into nucleus and cytochrome c into cytoplasm, but also inhibited the increase of Bax within mitochondria and alleviated the decrease of mitochondrial Bcl-2. Our study indicates that the protection of GRb1 on OGD-induced apoptosis in SH-SY5Y cells is associated with its protection on mitochondrial function and inhibition of release of AIF and cytochrome c.
Figures



Similar articles
-
Inhibition of autophagy via activation of PI3K/Akt pathway contributes to the protection of ginsenoside Rb1 against neuronal death caused by ischemic insults.Int J Mol Sci. 2014 Sep 1;15(9):15426-42. doi: 10.3390/ijms150915426. Int J Mol Sci. 2014. PMID: 25257523 Free PMC article.
-
Ginsenosides Rb1 and Rg1 Protect Primary Cultured Astrocytes against Oxygen-Glucose Deprivation/Reoxygenation-Induced Injury via Improving Mitochondrial Function.Int J Mol Sci. 2019 Dec 3;20(23):6086. doi: 10.3390/ijms20236086. Int J Mol Sci. 2019. PMID: 31816825 Free PMC article.
-
Protective effects of ginsenoside Rb(3) on oxygen and glucose deprivation-induced ischemic injury in PC12 cells.Acta Pharmacol Sin. 2010 Mar;31(3):273-80. doi: 10.1038/aps.2010.9. Epub 2010 Feb 8. Acta Pharmacol Sin. 2010. PMID: 20140005 Free PMC article.
-
Hemin protects against oxygen-glucose deprivation-induced apoptosis activation via neuroglobin in SH-SY5Y cells.Neurochem Res. 2017 Aug;42(8):2208-2217. doi: 10.1007/s11064-017-2230-z. Epub 2017 Mar 18. Neurochem Res. 2017. PMID: 28316021
-
Ginsenoside Re protects methamphetamine-induced mitochondrial burdens and proapoptosis via genetic inhibition of protein kinase C δ in human neuroblastoma dopaminergic SH-SY5Y cell lines.J Appl Toxicol. 2015 Aug;35(8):927-44. doi: 10.1002/jat.3093. Epub 2014 Dec 18. J Appl Toxicol. 2015. PMID: 25523949
Cited by
-
Inhibition of autophagy via activation of PI3K/Akt pathway contributes to the protection of ginsenoside Rb1 against neuronal death caused by ischemic insults.Int J Mol Sci. 2014 Sep 1;15(9):15426-42. doi: 10.3390/ijms150915426. Int J Mol Sci. 2014. PMID: 25257523 Free PMC article.
-
Ginsenoside Rb1 reduced ischemic stroke-induced apoptosis through endoplasmic reticulum stress-associated IRE1/TRAF2/JNK pathway.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jan;398(1):747-764. doi: 10.1007/s00210-024-03292-4. Epub 2024 Jul 25. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39052059
-
SNHG12 inhibits oxygen‑glucose deprivation‑induced neuronal apoptosis via the miR‑181a‑5p/NEGR1 axis.Mol Med Rep. 2020 Nov;22(5):3886-3894. doi: 10.3892/mmr.2020.11459. Epub 2020 Aug 24. Mol Med Rep. 2020. PMID: 33000228 Free PMC article.
-
Neuroprotective Effects of Ginseng Phytochemicals: Recent Perspectives.Molecules. 2019 Aug 14;24(16):2939. doi: 10.3390/molecules24162939. Molecules. 2019. PMID: 31416121 Free PMC article. Review.
-
Evaluation of the adaptogenic potential exerted by ginsenosides Rb1 and Rg1 against oxidative stress-mediated neurotoxicity in an in vitro neuronal model.PLoS One. 2017 Aug 16;12(8):e0182933. doi: 10.1371/journal.pone.0182933. eCollection 2017. PLoS One. 2017. PMID: 28813475 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

