Synthesis of cycloveratrylene macrocycles and benzyl oligomers catalysed by bentonite under microwave/infrared and solvent-free conditions
- PMID: 24135939
- PMCID: PMC6269697
- DOI: 10.3390/molecules181012820
Synthesis of cycloveratrylene macrocycles and benzyl oligomers catalysed by bentonite under microwave/infrared and solvent-free conditions
Abstract
Tonsil Actisil FF, which is a commercial bentonitic clay, promotes the formation of cycloveratrylene macrocycles and benzyl oligomers from the corresponding benzyl alcohols in good yields under microwave heating and infrared irradiation in the absence of solvent in both cases. The catalytic reaction is sensitive to the type of substituent on the aromatic ring. Thus, when benzyl alcohol was substituted with a methylenedioxy, two methoxy or three methoxy groups, a cyclooligomerisation process was induced. Unsubstituted, methyl and methoxy benzyl alcohols yielded linear oligomers. In addition, computational chemistry calculations were performed to establish a validated mechanistic pathway to explain the growth of the obtained linear oligomers.
Figures
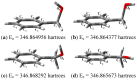



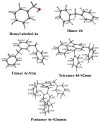

References
-
- Taylor F.R.S., Sá J., Hardacre C. Friedel–Crafts alkylation of aromatics with benzyl alcohol over gold-modified silica. ChemCatChem. 2011;3:119–121. doi: 10.1002/cctc.201000337. - DOI
-
- Salmón M., Miranda R., Nicolás-Vázquez I., Vargas-Rodriguez M.Y., Cruz-Borbolla J., Medrano M.I., Morales-Serna J.A. Effects of bentonite on p-methoxybenzyl acetate: A theoretical model for oligomerization via an electrophilic-substitution mechanism. Molecules. 2011;16:1761–1775. doi: 10.3390/molecules16021761. - DOI - PMC - PubMed
-
- Cruz-Almanza R., Shiba-Matzumoto I., Fuentes A., Martínez M., Cabrera A., Cárdenas J., Salmon M. Oligomerization of benzylic alcohols and its mechanism. J. Mol. Catal. A. 1997;126:161–168.
-
- Peterca M., Percec V., Imam M.R., Leowanawat P., Morimitsu K., Heiney P.A. Molecular structure of helical supramolecular dendrimers. J. Am. Chem. Soc. 2008;130:14840–14852. - PubMed
-
- Miranda R., Rios H., Delgado F., Castro M., Cogordan A., Salmón M. Characterization of a bentonitic clay and its application as catalyst in the preparation of benzyltoluenes and oligotoluenes. Appl. Catal. A. 2003;244:217–233. doi: 10.1016/S0926-860X(02)00566-5. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

