The genetic basis of myelodysplasia and its clinical relevance
- PMID: 24136165
- PMCID: PMC3862275
- DOI: 10.1182/blood-2013-09-381665
The genetic basis of myelodysplasia and its clinical relevance
Abstract
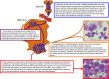
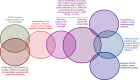
Myelodysplasia is a diagnostic feature of myelodysplastic syndromes (MDSs) but is also found in other myeloid neoplasms. Its molecular basis has been recently elucidated by means of massive parallel sequencing studies. About 90% of MDS patients carry ≥1 oncogenic mutations, and two thirds of them are found in individuals with a normal karyotype. Driver mutant genes include those of RNA splicing (SF3B1, SRSF2, U2AF1, and ZRSR2), DNA methylation (TET2, DNMT3A, and IDH1/2), chromatin modification (ASXL1 and EZH2), transcription regulation (RUNX1), DNA repair (TP53), signal transduction (CBL, NRAS, and KRAS), and cohesin complex (STAG2). Only 4 to 6 genes are consistently mutated in ≥10% MDS patients, whereas a long tail of ∼50 genes are mutated less frequently. At presentation, most patients typically have 2 or 3 driver oncogenic mutations and hundreds of background mutations. MDS driver genes are also frequently mutated in other myeloid neoplasms. Reliable genotype/phenotype relationships include the association of the SF3B1 mutation with refractory anemia with ring sideroblasts, TET2/SRSF2 comutation with chronic myelomonocytic leukemia, and activating CSF3R mutation with chronic neutrophilic leukemia. Although both founding and subclonal driver mutations have been shown to have prognostic significance, prospective clinical trials that include the molecular characterization of the patient's genome are now needed.
Figures



References
-
- Swerdlow SH, Campo E, Harris NL, et al. Lyon, France: IARC; 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues.
-
- Lindsley RC, Ebert BL. The biology and clinical impact of genetic lesions in myeloid malignancies [published online ahead of print August 16, 2013]. Blood. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

