The G-protein-coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian cancer cells by blocking tubulin polymerization
- PMID: 24136233
- PMCID: PMC3920961
- DOI: 10.1038/cddis.2013.397
The G-protein-coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian cancer cells by blocking tubulin polymerization
Abstract
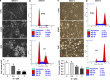
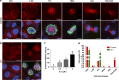
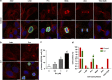
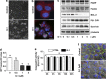
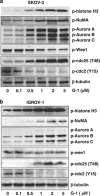
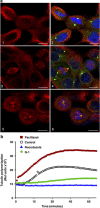
The G-protein-coupled estrogen receptor 1 (GPER) has recently been reported to mediate the non-genomic action of estrogen in different types of cells and tissues. G-1 (1-[4-(6-bromobenzo[1,3] dioxol-5yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone) was developed as a potent and selective agonist for GPER. G-1 has been shown to induce the expression of genes and activate pathways that facilitate cancer cell proliferation by activating GPER. Here we demonstrate that G-1 has an anticancer potential with a mechanism similar to vinca alkaloids, the commonly used chemotherapy drugs. We found that G-1 blocks tubulin polymerization and thereby interrupts microtubule assembly in ovarian cancer cells leading to the arrest of cell cycle in the prophase of mitosis and the suppression of ovarian cancer cell proliferation. G-1 treatment also induces apoptosis of ovarian cancer cells. The ability of G-1 to target microtubules to suppress ovarian cancer cell proliferation makes it a promising candidate drug for treatment of ovarian cancer.
Figures

References
-
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

