Once-daily fluticasone furoate alone or combined with vilanterol in persistent asthma
- PMID: 24136330
- PMCID: PMC3938760
- DOI: 10.1183/09031936.00064513
Once-daily fluticasone furoate alone or combined with vilanterol in persistent asthma
Abstract
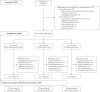
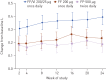
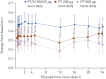
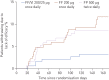
The inhaled corticosteroid fluticasone furoate (FF) and the long-acting β₂ agonist vilanterol (VI) are in development as a combined once-daily therapy for asthma and chronic obstructive pulmonary disease. Our study objectives were to compare the efficacy and safety of once-daily FF/VI with FF alone and twice-daily fluticasone propionate (FP) in patients aged ≥12 years with moderate-to-severe persistent asthma. Patients (n=586) received FF/VI 200/25 μg or FF 200 μg once-daily (evening dosing), or FP 500 μg twice-daily for 24 weeks. Co-primary end-points were change from baseline in trough forced expiratory volume in 1 s (FEV₁) weighted mean (wm) 0-24 h serial FEV1. Secondary end-points included change from baseline in percentage of rescue-free 24-h periods, percentage of symptom-free 24-h periods and total Asthma Quality of Life Questionnaire (AQLQ). Safety assessments included adverse events, 24-h urinary cortisol excretion, vital signs and ECG. FF/VI significantly improved trough FEV1 and wmFEV₁ versus FF and FP. Significantly more rescue-free and symptom-free 24-h periods were reported with FF/VI versus FF. Treatment differences for AQLQ were not significant. Incidence of adverse events was similar across groups. No clinically significant differences were seen for 24-h urinary cortisol excretion, vital signs or ECG. FF/VI resulted in statistically greater improvements in lung function and symptomatic end-points versus FF, and was well tolerated in this asthma population.
Trial registration: ClinicalTrials.gov NCT01134042.
Conflict of interest statement
Conflict of interest: Disclosures can be found alongside the online version of this article at
Figures
References
-
- British Thoracic Society and Scottish Intercollegiate Guidelines Network British guideline on the management of asthma: a national clinical guideline. Thorax 2008; 63: Suppl. 4, iv1–iv121 - PubMed
-
- Global Initiative for Asthma Global Strategy for Asthma Management and Prevention, 2012. www.ginasthma.org/documents/4 Date last updated: December, 2012. Date last accessed: October 9, 2013
-
- Fanta CH. Drug therapy: asthma. N Engl J Med 2009; 360: 1002–1014 - PubMed
-
- US prescribing information for veramyst (fluticasone furoate) nasal spray Indicated for treatment of symptoms of seasonal and perennial allergic rhinitis in adults and children ≥2 years. www.accessdata.fda.gov/drugsatfda_docs/label/2011/022051s007lbl.pdf Date last updated: October, 2011. Date last accessed: October 9, 2013
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
