Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage
- PMID: 24136356
- PMCID: PMC3930011
- DOI: 10.1126/science.1243292
Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage
Abstract
Genetic mutations cause primary immunodeficiencies (PIDs) that predispose to infections. Here, we describe activated PI3K-δ syndrome (APDS), a PID associated with a dominant gain-of-function mutation in which lysine replaced glutamic acid at residue 1021 (E1021K) in the p110δ protein, the catalytic subunit of phosphoinositide 3-kinase δ (PI3Kδ), encoded by the PIK3CD gene. We found E1021K in 17 patients from seven unrelated families, but not among 3346 healthy subjects. APDS was characterized by recurrent respiratory infections, progressive airway damage, lymphopenia, increased circulating transitional B cells, increased immunoglobulin M, and reduced immunoglobulin G2 levels in serum and impaired vaccine responses. The E1021K mutation enhanced membrane association and kinase activity of p110δ. Patient-derived lymphocytes had increased levels of phosphatidylinositol 3,4,5-trisphosphate and phosphorylated AKT protein and were prone to activation-induced cell death. Selective p110δ inhibitors IC87114 and GS-1101 reduced the activity of the mutant enzyme in vitro, which suggested a therapeutic approach for patients with APDS.
Figures
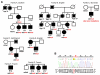
 and
and  - available data indicate recurrent infections. Age at the time of death is shown for patients who died ≤30 years of age. PIK3CD genotype is shown if known: wt, wild type allele encoding glutamic acid (E1021); mut, mutant allele encoding lysine (K1021). (B) Sequence chromatogram showing heterozygous mutation c.3061G>A in the PIK3CD gene leading to the E1021K amino-acid change in p110δ. CpG dinucleotide is underlined.
- available data indicate recurrent infections. Age at the time of death is shown for patients who died ≤30 years of age. PIK3CD genotype is shown if known: wt, wild type allele encoding glutamic acid (E1021); mut, mutant allele encoding lysine (K1021). (B) Sequence chromatogram showing heterozygous mutation c.3061G>A in the PIK3CD gene leading to the E1021K amino-acid change in p110δ. CpG dinucleotide is underlined.
Comment in
-
Genetics. Can cancer drugs treat immunodeficiency?Science. 2013 Nov 15;342(6160):814-5. doi: 10.1126/science.1246760. Science. 2013. PMID: 24233715 No abstract available.
References
-
- Barker AF. Bronchiectasis. N Engl J Med. 2002;346:1383. - PubMed
-
- Durandy A, Kracker S, Fischer A. Primary antibody deficiencies. Nat Rev Immunol. 2013;13:519. - PubMed
-
- Bamshad MJ, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12:745. - PubMed
-
-
Materials and methods are available as supplementary materials on Science online.
-
Publication types
MeSH terms
Substances
Grants and funding
- 095198/Z/10/Z/WT_/Wellcome Trust/United Kingdom
- 095691/WT_/Wellcome Trust/United Kingdom
- BBS/E/B/000C0411/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J004456/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- U105184308/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

