Biodistribution and radiation dosimetry of 18F-CP-18, a potential apoptosis imaging agent, as determined from PET/CT scans in healthy volunteers
- PMID: 24136934
- PMCID: PMC7607624
- DOI: 10.2967/jnumed.113.119800
Biodistribution and radiation dosimetry of 18F-CP-18, a potential apoptosis imaging agent, as determined from PET/CT scans in healthy volunteers
Abstract
(18)F-CP-18, or (18S,21S,24S,27S,30S)-27-(2-carboxyethyl)-21-(carboxymethyl)-30-((2S,3R,4R,5R,6S)-6-((2-(4-(3-F18-fluoropropyl)-1H-1,2,3-triazol-1-yl)acetamido)methyl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-carboxamido)-24-isopropyl-18-methyl-17,20,23,26,29-pentaoxo-4,7,10,13-tetraoxa-16,19,22,25,28-pentaazadotriacontane-1,32-dioic acid, is being evaluated as a tissue apoptosis marker for PET imaging. The purpose of this study was to determine the biodistribution and estimate the normal-organ radiation-absorbed doses and effective dose from (18)F-CP-18.
Methods: Successive whole-body PET/CT scans were obtained at approximately 7, 45, 90, 130, and 170 min after intravenous injection of (18)F-CP-18 in 7 healthy human volunteers. Blood samples and urine were collected between the PET/CT scans, and the biostability of (18)F-CP-18 was assessed using high-performance liquid chromatography. The PET scans were analyzed to determine the radiotracer uptake in different organs. OLINDA/EXM software was used to calculate human radiation doses based on the biodistribution of the tracer.
Results: (18)F-CP-18 was 54% intact in human blood at 135 min after injection. The tracer cleared rapidly from the blood pool with a half-life of approximately 30 min. Relatively high (18)F-CP-18 uptake was observed in the kidneys and bladder, with diffuse uptake in the liver and heart. The mean standardized uptake values (SUVs) in the bladder, kidneys, heart, and liver at around 50 min after injection were approximately 65, 6, 1.5, and 1.5, respectively. The calculated effective dose was 38 ± 4 μSv/MBq, with the urinary bladder wall having the highest absorbed dose at 536 ± 61 μGy/MBq using a 4.8-h bladder-voiding interval for the male phantom. For a 1-h voiding interval, these doses were reduced to 15 ± 2 μSv/MBq and 142 ± 15 μGy/MBq, respectively. For a typical injected activity of 555 MBq, the effective dose would be 21.1 ± 2.2 mSv for the 4.8-h interval, reduced to 8.3 ± 1.1 mSv for the 1-h interval.
Conclusion: (18)F-CP-18 cleared rapidly through the renal system. The urinary bladder wall received the highest radiation dose and was deemed the critical organ. Both the effective dose and the bladder dose can be reduced by frequent voiding. From the radiation dosimetry perspective, the apoptosis imaging agent (18)F-CP-18 is suitable for human use.
Trial registration: ClinicalTrials.gov NCT01362712.
Keywords: 18F-CP-18; PET; apoptosis marker; biodistribution; internal dosimetry.
Figures
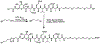


Similar articles
-
Biodistribution and radiation dosimetry of the integrin marker 18F-RGD-K5 determined from whole-body PET/CT in monkeys and humans.J Nucl Med. 2012 May;53(5):787-95. doi: 10.2967/jnumed.111.088955. Epub 2012 Apr 12. J Nucl Med. 2012. PMID: 22499613 Free PMC article. Clinical Trial.
-
Biodistribution and radiation dosimetry of the hypoxia marker 18F-HX4 in monkeys and humans determined by using whole-body PET/CT.Nucl Med Commun. 2010 Dec;31(12):1016-24. doi: 10.1097/MNM.0b013e3283407950. Nucl Med Commun. 2010. PMID: 20948452 Free PMC article. Clinical Trial.
-
Biodistribution and radiation dosimetry of the carbonic anhydrase IX imaging agent [(18) F]VM4-037 determined from PET/CT scans in healthy volunteers.Mol Imaging Biol. 2014 Oct;16(5):739-46. doi: 10.1007/s11307-014-0730-7. Mol Imaging Biol. 2014. PMID: 24696183 Free PMC article. Clinical Trial.
-
Noninvasive estimation of human radiation dosimetry of 18F-FDG by whole-body small animal PET imaging in rats.Appl Radiat Isot. 2022 Mar;181:110071. doi: 10.1016/j.apradiso.2021.110071. Epub 2021 Dec 17. Appl Radiat Isot. 2022. PMID: 34952332 Review.
-
18F-NaF PET/CT of Obese Patients on a Lutetium-Yttrium Oxyorthosilicate PET/CT System: Patient Dosimetry, Optimization of Injected Activity, and Acquisition Time.J Nucl Med Technol. 2021 Jun;49(2):150-155. doi: 10.2967/jnmt.120.258137. Epub 2020 Dec 30. J Nucl Med Technol. 2021. PMID: 33380519 Review.
Cited by
-
Avenues to molecular imaging of dying cells: Focus on cancer.Med Res Rev. 2018 Sep;38(6):1713-1768. doi: 10.1002/med.21495. Epub 2018 Mar 12. Med Res Rev. 2018. PMID: 29528513 Free PMC article. Review.
-
MSCs-engineered biomimetic PMAA nanomedicines for multiple bioimaging-guided and photothermal-enhanced radiotherapy of NSCLC.J Nanobiotechnology. 2021 Mar 20;19(1):80. doi: 10.1186/s12951-021-00823-6. J Nanobiotechnology. 2021. PMID: 33743720 Free PMC article.
-
Radionuclide imaging of apoptosis for clinical application.Eur J Nucl Med Mol Imaging. 2022 Mar;49(4):1345-1359. doi: 10.1007/s00259-021-05641-4. Epub 2021 Dec 7. Eur J Nucl Med Mol Imaging. 2022. PMID: 34873639 Free PMC article. Review.
-
First-in-human study of a novel cell death tracer [99mTc]Tc-Duramycin: safety, biodistribution and radiation dosimetry in healthy volunteers.EJNMMI Radiopharm Chem. 2023 Aug 30;8(1):20. doi: 10.1186/s41181-023-00207-1. EJNMMI Radiopharm Chem. 2023. PMID: 37646865 Free PMC article.
-
11C Dosimetry Scans Should Be Abandoned.J Nucl Med. 2021 Feb;62(2):158-159. doi: 10.2967/jnumed.120.257402. Epub 2020 Dec 11. J Nucl Med. 2021. PMID: 33310737 Free PMC article. No abstract available.
References
-
- Graeber TG, Osmanian C, Jacks T, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. - PubMed
-
- Reshef A, Shirvan A, Waterhouse RN, et al. Molecular imaging of neurovascular cell death in experimental cerebral stroke by PET. J Nucl Med. 2008;49:1520–1528. - PubMed
-
- Cohen A, Shirvan A, Levin G, Grimberg H, Reshef A, Ziv I. From the Gla domain to a novel small-molecule detector of apoptosis. Cell Res. 2009;19:625–637. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
