Diversity of layer 5 projection neurons in the mouse motor cortex
- PMID: 24137110
- PMCID: PMC3797544
- DOI: 10.3389/fncel.2013.00174
Diversity of layer 5 projection neurons in the mouse motor cortex
Abstract
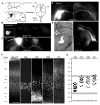
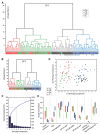
In the primary motor cortex (M1), layer 5 projection neurons signal directly to distant motor structures to drive movement. Despite their pivotal position and acknowledged diversity these neurons are traditionally separated into broad commissural and corticofugal types, and until now no attempt has been made at resolving the basis for their diversity. We therefore probed the electrophysiological and morphological properties of retrogradely labeled M1 corticospinal (CSp), corticothalamic (CTh), and commissural projecting corticostriatal (CStr) and corticocortical (CC) neurons. An unsupervised cluster analysis established at least four phenotypes with additional differences between lumbar and cervical projecting CSp neurons. Distinguishing parameters included the action potential (AP) waveform, firing behavior, the hyperpolarisation-activated sag potential, sublayer position, and soma and dendrite size. CTh neurons differed from CSp neurons in showing spike frequency acceleration and a greater sag potential. CStr neurons had the lowest AP amplitude and maximum rise rate of all neurons. Temperature influenced spike train behavior in corticofugal neurons. At 26°C CTh neurons fired bursts of APs more often than CSp neurons, but at 36°C both groups fired regular APs. Our findings provide reliable phenotypic fingerprints to identify distinct M1 projection neuron classes as a tool to understand their unique contributions to motor function.
Keywords: cluster analysis; corticocortical; corticospinal; corticostriatal; corticothalamic; electrophysiology; morphology.
Figures





References
-
- Amitai Y., Connors B. W. (1995). “ Intrinsic physiology and morphology of single neurons in neocortex,” in Cerebral Cortex eds Jones E. G., Diamond I. T. (New York: Plenum Press; ) 299–331
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

