Stress affects theta activity in limbic networks and impairs novelty-induced exploration and familiarization
- PMID: 24137113
- PMCID: PMC3797543
- DOI: 10.3389/fnbeh.2013.00127
Stress affects theta activity in limbic networks and impairs novelty-induced exploration and familiarization
Abstract
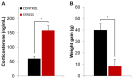
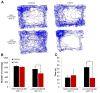
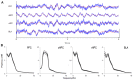
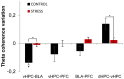
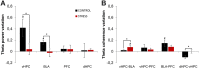
Exposure to a novel environment triggers the response of several brain areas that regulate emotional behaviors. Here, we studied theta oscillations within the hippocampus (HPC)-amygdala (AMY)-medial prefrontal cortex (mPFC) network in exploration of a novel environment and subsequent familiarization through repeated exposures to that same environment; in addition, we assessed how concomitant stress exposure could disrupt this activity and impair both behavioral processes. Local field potentials (LFP) were simultaneously recorded from dorsal and ventral hippocampus (dHPC and vHPC, respectively), basolateral amygdala (BLA) and mPFC in freely behaving rats while they were exposed to a novel environment, then repeatedly re-exposed over the course of 3 weeks to that same environment and, finally, on re-exposure to a novel unfamiliar environment. A longitudinal analysis of theta activity within this circuit revealed a reduction of vHPC and BLA theta power and vHPC-BLA theta coherence through familiarization which was correlated with a return to normal exploratory behavior in control rats. In contrast, a persistent over-activation of the same brain regions was observed in stressed rats that displayed impairments in novel exploration and familiarization processes. Importantly, we show that stress also affected intra-hippocampal synchrony and heightened the coherence between vHPC and BLA. In summary, we demonstrate that modulatory theta activity in the aforementioned circuit, namely in the vHPC and BLA, is correlated with the expression of anxiety in novelty-induced exploration and familiarization in both normal and pathological conditions.
Keywords: amygdala; anxiety; local field potentials; pre-frontal cortex; stress; ventral hippocampus.
Figures


References
Supplemental reference
-
- Paxinos G., Watson C. (2006). The Rat Brain in Stereotaxic Coordinates, 6th Edn. London: Elsevier
References
-
- Behrendt R. F. (2011). Neuroanatomy of Social Behaviour: an Evolutionary and Psychoanalytic Perspective. London: Karnac
LinkOut - more resources
Full Text Sources
Other Literature Sources

