CD90(+) Stromal Cells are Non-Professional Innate Immune Effectors of the Human Colonic Mucosa
- PMID: 24137162
- PMCID: PMC3786311
- DOI: 10.3389/fimmu.2013.00307
CD90(+) Stromal Cells are Non-Professional Innate Immune Effectors of the Human Colonic Mucosa
Erratum in
-
Corrigendum: CD90(+) Stromal Cells are Non-Professional Innate Immune Effectors of the Human Colonic Mucosa.Front Immunol. 2015 Jun 24;6:325. doi: 10.3389/fimmu.2015.00325. eCollection 2015. Front Immunol. 2015. PMID: 26157441 Free PMC article.
Abstract
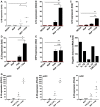
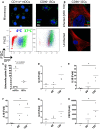
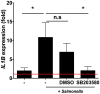
Immune responses at the intestinal mucosa must allow for host protection whilst simultaneously avoiding inappropriate inflammation. Although much work has focused on the innate immune functionality of hematopoietic immune cells, non-hematopoietic cell populations - including epithelial and stromal cells - are now recognized as playing a key role in innate defense at this site. In this study we examined the innate immune capacity of primary human intestinal stromal cells (iSCs). CD90(+) iSCs isolated from human colonic mucosa expressed a wide array of innate immune receptors and functionally responded to stimulation with bacterial ligands. iSCs also sensed infection with live Salmonella typhimurium, rapidly expressing IL-1 family cytokines via a RIPK2/p38MAPK-dependent signaling process. In addition to responding to innate immune triggers, primary iSCs exhibited a capacity for bacterial uptake, phagocytosis, and antigen processing, although to a lesser extent than professional APCs. Thus CD90(+) iSCs represent an abundant population of "non-professional" innate immune effector cells of the human colonic mucosa and likely play an important adjunctive role in host defense and immune regulation at this site.
Keywords: innate immunity; intestinal homeostasis; mucosal immunology; stromal cells.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

