Isolation and characterization of serum albumin from Camelus dromedarius
- PMID: 24137219
- PMCID: PMC3786902
- DOI: 10.3892/etm.2013.1145
Isolation and characterization of serum albumin from Camelus dromedarius
Abstract
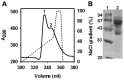
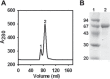
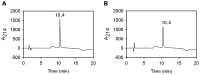
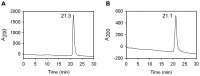
Serum albumin constitutes 35-50 mg/ml of plasma proteins and performs various physiological activities including the regulation of osmotic pressure on blood, maintaining buffering of the blood pH, carrying different fatty acids and other small molecules, such as bilirubin, hormones, drugs and metal ions, as well as participating in immunological responses. Serum albumin is an extensively used protein in biotechnological and pharmaceutical industries. The camel (Camelus dromedarius) is well tailored to successfully survive in extremely hot and dry climates. Plasma osmolality in the camel increases during water-deprived conditions. In such circumstances serum albumin is crucial in the regulation of blood pressure. The study of biochemical, biophysical and immunological aspects of camel serum albumin (CSA) are likely to provide molecular insights into camel physiology and may render it an alternative to human serum albumin (HSA) and bovine serum albumin (BSA) in all cases. However, these proteins are currently not available or cannot be utilized due to a variety of considerations. In this study, 12 mg of highly pure CSA was obtained from 1 ml plasma. Coomassie Brilliant Blue staining of SDS-PAGE yielded one band and RP-HPLC results revealed a single sharp peak, indicating homogenous preparation of the CSA. The charge/mass ratio and surface hydrophobicity of the CSA was similar to that of BSA. Mass spectrometry analysis of the purified protein confirmed the identity of CSA.
Keywords: camel serum albumin; capillary electrophoresis; high-performance liquid chromatography; protein purification and characterization.
Figures


Similar articles
-
Heat-induced conformational unfolding of fatty acid-free and fatted camel serum albumin: a comparative study.Cell Biochem Biophys. 2013 Nov;67(2):727-34. doi: 10.1007/s12013-013-9562-3. Cell Biochem Biophys. 2013. PMID: 23512227
-
Purification and biochemical characterization of a novel copper, zinc superoxide dismutase from liver of camel (Camelus dromedarius): An antioxidant enzyme with unique properties.Bioorg Chem. 2019 May;86:428-436. doi: 10.1016/j.bioorg.2019.02.024. Epub 2019 Feb 11. Bioorg Chem. 2019. PMID: 30771689
-
Purification and functional characterization of pancreatic insulin from camel (Camelus dromedarius).Saudi J Biol Sci. 2014 Dec;21(6):574-81. doi: 10.1016/j.sjbs.2014.03.001. Epub 2014 Mar 29. Saudi J Biol Sci. 2014. PMID: 25473366 Free PMC article.
-
Novel insights into the pleiotropic effects of human serum albumin in health and disease.Biochim Biophys Acta. 2013 Dec;1830(12):5486-93. doi: 10.1016/j.bbagen.2013.04.012. Epub 2013 Apr 17. Biochim Biophys Acta. 2013. PMID: 23602811 Review.
-
Recent developments in the detection of bovine serum albumin.Int J Biol Macromol. 2019 Oct 1;138:602-617. doi: 10.1016/j.ijbiomac.2019.07.096. Epub 2019 Jul 15. Int J Biol Macromol. 2019. PMID: 31319084 Review.
Cited by
-
Oxidation of human serum albumin exhibits inter-individual variability after an ultra-marathon mountain race.Exp Ther Med. 2017 May;13(5):2382-2390. doi: 10.3892/etm.2017.4268. Epub 2017 Mar 28. Exp Ther Med. 2017. PMID: 28565852 Free PMC article.
-
Purification and Characterization of Bovine Serum Albumin Using Chromatographic Method.Adv Pharm Bull. 2016 Dec;6(4):651-654. doi: 10.15171/apb.2016.080. Epub 2016 Dec 22. Adv Pharm Bull. 2016. PMID: 28101473 Free PMC article.
-
The Use of Human, Bovine, and Camel Milk Albumins in Anticancer Complexes with Oleic Acid.Protein J. 2018 Jun;37(3):203-215. doi: 10.1007/s10930-018-9770-1. Protein J. 2018. PMID: 29691701
References
-
- Lundsgaard-Hansen P. Physiology and pathophysiology of colloid osmotic pressure and albumin metabolism. Curr Stud Hematol Blood Transfus. 1986;53:1–17. - PubMed
-
- Prajapati KD, Sharma SS, Roy N. Current perspectives on potential role of albumin in neuroprotection. Rev Neurosci. 2011;22:355–363. - PubMed
-
- Georgiou HM, Rice GE, Baker MS. Proteomic analysis of human plasma: failure of centrifugal ultrafiltration to remove albumin and other high molecular weight proteins. Proteomics. 2001;1:1503–1506. - PubMed
-
- Jiang L, He L, Fountoulakis M. Comparison of protein precipitation methods for sample preparation prior to proteomic analysis. J Chromatogr A. 2004;1023:317–320. - PubMed
-
- Olver CS, Webb TL, Long LJ, Scherman H, Prenni JE. Comparison of methods for depletion of albumin and IgG from equine serum. Vet Clin Pathol. 2010;39:337–345. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
