Construction and expression of D-dimer and GPIIb/IIIa single-chain bispecific antibody
- PMID: 24137225
- PMCID: PMC3786976
- DOI: 10.3892/etm.2013.1132
Construction and expression of D-dimer and GPIIb/IIIa single-chain bispecific antibody
Abstract
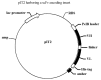
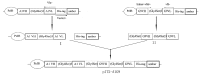
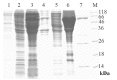
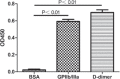
The aim of this study was to construct a plasmid expressing glycoprotein IIb-IIIa (GPIIb/IIIa) and D-dimer single-chain bispecific antibody for the targeted therapy of thrombosis. The phosphorylated gene encoding the anti-GPIIb/IIIa single-chain variable fragment (scFv) and the gene encoding the anti-D-dimer scFv were amplified by PCR and linked in tandem by blunt-end ligation. The recombinant plasmid was transfected into the competent cell line HB2151 and identified by PCR and DNA sequencing. Then, the soluble recombinant antibody in bacterial lysates was purified by an NTA column and molecular sieve chromatography in turn. Finally, the binding specificity of the purified antibody was tested by enzyme-linked immunosorbent assay (ELISA). Results demonstrated that the construction of the expression plasmid was successful and the purified recombinant protein, which had a molecular weight of ∼56 kDa, was specific to GPIIb/IIIa and D-dimer. In conclusion, a plasmid expressing a bispecific antibody was constructed by a new method of blunt-end ligation. The soluble recombinant protein is a promising platform for target-oriented thrombolytic therapy.
Keywords: D-dimer; glycoprotein IIb-IIIa; single-chain bispecific antibody.
Figures



Similar articles
-
[Construction and clinical applications of a humanized phage library of platelet GPIIb/IIIa single chain Fv antibody].Zhonghua Nei Ke Za Zhi. 2005 Apr;44(4):293-6. Zhonghua Nei Ke Za Zhi. 2005. PMID: 15924647 Chinese.
-
[Construction and characterization of bispecific single-chain antibody fragments SZ-2/SZ-21 against platelet glycoprotein Ib alpha and beta3].Zhonghua Yi Xue Za Zhi. 2002 Nov 10;82(21):1493-7. Zhonghua Yi Xue Za Zhi. 2002. PMID: 12509914 Chinese.
-
Screening and evaluation of human single-chain fragment variable antibody against hepatitis B virus surface antigen.Hepatobiliary Pancreat Dis Int. 2006 May;5(2):237-41. Hepatobiliary Pancreat Dis Int. 2006. PMID: 16698583
-
Clues for understanding the structure and function of a prototypic human integrin: the platelet glycoprotein IIb/IIIa complex.Thromb Haemost. 1994 Jul;72(1):1-15. Thromb Haemost. 1994. PMID: 7974356 Review.
-
Overcoming thrombolytic resistance: rationale and initial clinical experience combining thrombolytic therapy and glycoprotein IIb/IIIa receptor inhibition for acute myocardial infarction.J Am Coll Cardiol. 1999 Nov 1;34(5):1395-402. doi: 10.1016/s0735-1097(99)00364-2. J Am Coll Cardiol. 1999. PMID: 10551684 Review.
Cited by
-
Use of Epivolve phage display to generate a monoclonal antibody with opsonic activity directed against a subdominant epitope on extracellular loop 4 of Treponema pallidum BamA (TP0326).Front Immunol. 2023 Aug 22;14:1222267. doi: 10.3389/fimmu.2023.1222267. eCollection 2023. Front Immunol. 2023. PMID: 37675118 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
