Inhibition of hepatocellular carcinoma growth by adenovirus-mediated expression of human telomerase reverse transcriptase COOH-27 terminal polypeptide in mice
- PMID: 24137404
- PMCID: PMC3789117
- DOI: 10.3892/ol.2013.1470
Inhibition of hepatocellular carcinoma growth by adenovirus-mediated expression of human telomerase reverse transcriptase COOH-27 terminal polypeptide in mice
Abstract
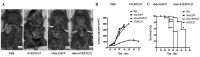
A 27-kDa C-terminal fragment of human telomerase reverse transcriptase, hTERTC27, has previously been reported to inhibit the growth and tumorigenicity of HeLa human cervical cancer cells and U87-MG human glioblastoma multiforme cells. However, the antitumor effects of hTERTC27 in hepatoma and its underlying mechanisms are unclear. In the current study, the therapeutic effect of hTERTC27, mediated by recombinant adenovirus, in hepatocellular carcinoma (HCC) was explored in vitro and in vivo to investigate the possible mechanisms. The results indicated that recombinant adenovirus carrying hTERTC27 (rAdv-hTERTC27) effectively inhibited the growth and induced apoptosis of the Hepa 1-6 HCC cells. Dendritic cells transduced with rAdv-hTERTC27 were highly effective at inducing antigen-specific T cell proliferation and increasing the activated cytotoxicity of T cells against Hepa 1-6 cells. HCC was inhibited significantly when a single dose of 5×107 pfu rAdv-hTERTC27 was administered intravenously. In summary, the results of this study demonstrated that rAdv-hTERTC27 may serve as a reagent for intravenous administration when combined with telomerase-based gene therapy and immunotherapy for cancer.
Keywords: cytotoxic T lymphocytes; gene therapy; hTERTC27; hepatocellular carcinoma; immunotherapy.
Figures

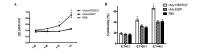
Similar articles
-
Suppressing tumor growth of nasopharyngeal carcinoma by hTERTC27 polypeptide delivered through adeno-associated virus plus adenovirus vector cocktail.Chin J Cancer. 2012 Dec;31(12):588-97. doi: 10.5732/cjc.011.10378. Epub 2012 Nov 13. Chin J Cancer. 2012. PMID: 23149313 Free PMC article.
-
Development of recombinant adeno-associated virus and adenovirus cocktail system for efficient hTERTC27 polypeptide-mediated cancer gene therapy.Cancer Gene Ther. 2008 Nov;15(11):723-32. doi: 10.1038/cgt.2008.33. Epub 2008 Jun 6. Cancer Gene Ther. 2008. PMID: 18535618
-
Inhibition of melanoma growth by subcutaneous administration of hTERTC27 viral cocktail in C57BL/6 mice.PLoS One. 2010 Sep 13;5(9):e12705. doi: 10.1371/journal.pone.0012705. PLoS One. 2010. PMID: 20856939 Free PMC article.
-
A novel glioblastoma cancer gene therapy using AAV-mediated long-term expression of human TERT C-terminal polypeptide.Cancer Gene Ther. 2007 Jun;14(6):561-72. doi: 10.1038/sj.cgt.7701038. Epub 2007 Mar 23. Cancer Gene Ther. 2007. PMID: 17384579
-
Effective antitumor immunity against murine gliomas using dendritic cells transduced with hTERTC27 recombinant adenovirus.Oncol Rep. 2012 Apr;27(4):1163-9. doi: 10.3892/or.2011.1619. Epub 2011 Dec 30. Oncol Rep. 2012. PMID: 22210010 Free PMC article.
Cited by
-
Cellular and molecular targets for the immunotherapy of hepatocellular carcinoma.Mol Cell Biochem. 2018 Jan;437(1-2):13-36. doi: 10.1007/s11010-017-3092-z. Epub 2017 Jun 7. Mol Cell Biochem. 2018. PMID: 28593566 Review.
References
-
- Fung SK, Lok AS. Management of patients with hepatitis B virus-induced cirrhosis. J Hepatol. 2005;42(Suppl 1):S54–S64. - PubMed
-
- Huang YH, Wu JC, Chen SC, Chen CH, Chiang JH, Huo TI, Lee PC, Chang FY, Lee SD. Survival benefit of transcatheter arterial chemoembolization in patients with hepatocellular carcinoma larger than 10 cm in diameter. Aliment Pharmacol Ther. 2006;23:129–135. - PubMed
-
- Ng KK, Poon RT. Current treatment strategy for hepatocellular carcinoma. Saudi Med J. 2007;28:1330–1338. - PubMed
-
- Makower D, Rozenblit A, Kaufman H, Edelman M, Lane ME, Zwiebel J, Haynes H, Wadler S. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin Cancer Res. 2003;9:693–702. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
