Aripiprazole treatment of irritability associated with autistic disorder and the relationship between prior antipsychotic exposure, adverse events, and weight change
- PMID: 24138011
- PMCID: PMC3804231
- DOI: 10.1089/cap.2012.0075
Aripiprazole treatment of irritability associated with autistic disorder and the relationship between prior antipsychotic exposure, adverse events, and weight change
Abstract
Objective: The purpose of this study was to evaluate the impact of prior antipsychotic exposure (PAE) on safety and tolerability outcomes in pediatric subjects receiving aripiprazole treatment.
Methods: This study was a post-hoc analysis of pooled data from two 8-week, double-blind, randomized, placebo-controlled studies evaluating aripiprazole for the treatment of irritability in pediatric subjects with autistic disorder, aged 6-17 years. Subjects were stratified by PAE; adverse events (AEs), and changes in weight, and metabolic measures were evaluated. For subjects receiving aripiprazole, regardless of PAE, baseline weight, age, gender, and symptom severity were evaluated in a regression model predicting body weight change.
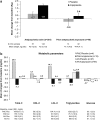
Results: Of 316 randomized subjects, 259 (82.0%) were antipsychotic naïve (AN) and 57 (18.0%) had a PAE. Aripiprazole-treated AN subjects were more likely than PAE subjects to report somnolence (11.9% vs. 2.8%), sedation (22.7% vs. 11.1%), or fatigue (17.0% vs. 13.9%). Rates of extrapyramidal disorder and drooling, but not akathisia or tremor, were marginally higher in AN subjects. Overall, 10.8% of aripiprazole-treated AN subjects had at least one AE leading to discontinuation compared with 8.3% of aripiprazole-treated PAE subjects. AN subjects receiving aripiprazole had a larger change in weight from baseline to endpoint compared with those receiving placebo (1.9 vs. 0.7 kg; treatment difference 1.2 kg, 95% CI: 0.5, 1.9) than PAE subjects receiving aripiprazole compared with subjects receiving placebo (0.4 vs. -0.4 kg; treatment difference 0.9 kg, 95% CI: -0.6, 2.4). Regression analysis identified that younger subjects with higher baseline weight z-score were at highest risk for weight gain. There were no significant changes in metabolic measures compared with placebo in either group.
Conclusions: Weight gain was more pronounced in AN subjects and more likely to occur in younger subjects with a higher baseline weight z-score. AN subjects were more likely to experience AEs related to somnolence. However, based on discontinuations rates from AEs, overall tolerability was good for both AN and PAE groups.
Clinical trial registration: Study of aripiprazole in the treatment of children and adolescents with autistic disorder. Registry: www.clinicaltrials.gov . Identifiers: NCT00332241 and NCT00337571.
Figures
References
-
- Aman MG. Singh NN. Aberrant Behavior Checklist: Manual. East Aurora, NY: Slosson Educational Publications; 1986.
-
- Guy W. Rockville, MD: National Institute of Mental Health; 1976. Clinical Global Impressions (CGI). ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare Publication (ADM) 76-338; pp. 218–222.
-
- Lord C. Rutter M. Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. - PubMed
-
- Marcus RN. Owen R. Kamen L. Manos G. McQuade RD. Carson WH. Aman MG. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:1110–1119. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

