Generation of highly purified neural stem cells from human adipose-derived mesenchymal stem cells by Sox1 activation
- PMID: 24138016
- PMCID: PMC3929335
- DOI: 10.1089/scd.2013.0263
Generation of highly purified neural stem cells from human adipose-derived mesenchymal stem cells by Sox1 activation
Abstract
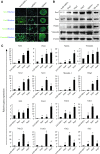
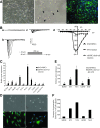
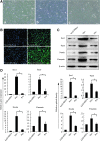
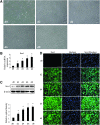
Neural stem cells (NSCs) are ideal candidates in stem cell-based therapy for neurodegenerative diseases. However, it is unfeasible to get enough quantity of NSCs for clinical application. Generation of NSCs from human adipose-derived mesenchymal stem cells (hAD-MSCs) will provide a solution to this problem. Currently, the differentiation of hAD-MSCs into highly purified NSCs with biological functions is rarely reported. In our study, we established a three-step NSC-inducing protocol, in which hAD-MSCs were induced to generate NSCs with high purity after sequentially cultured in the pre-inducing medium (Step1), the N2B27 medium (Step2), and the N2B27 medium supplement with basic fibroblast growth factor and epidermal growth factor (Step3). These hAD-MSC-derived NSCs (adNSCs) can form neurospheres and highly express Sox1, Pax6, Nestin, and Vimentin; the proportion was 96.1% ± 1.3%, 96.8% ± 1.7%, 96.2% ± 1.3%, and 97.2% ± 2.5%, respectively, as detected by flow cytometry. These adNSCs can further differentiate into astrocytes, oligodendrocytes, and functional neurons, which were able to generate tetrodotoxin-sensitive sodium current. Additionally, we found that the neural differentiation of hAD-MSCs were significantly suppressed by Sox1 interference, and what's more, Step1 was a key step for the following induction, probably because it was associated with the initiation and nuclear translocation of Sox1, an important transcriptional factor for neural development. Finally, we observed that bone morphogenetic protein signal was inhibited, and Wnt/β-catenin signal was activated during inducing process, and both signals were related with Sox1 expression. In conclusion, we successfully established a three-step inducing protocol to derive NSCs from hAD-MSCs with high purity by Sox1 activation. These findings might enable to acquire enough autologous transplantable NSCs for the therapy of neurodegenerative diseases in clinic.
Figures





References
-
- Honig LS. and Rosenberg RN. (2000). Apoptosis and neurologic disease. Am J Med 108:317–330 - PubMed
-
- Mujtaba T, Piper DR, Kalyani A, Groves AK, Lucero MT. and Rao MS. (1999). Lineage-restricted neural precursors can be isolated from both the mouse neural tube and cultured ES cells. Dev Biol 214:113–127 - PubMed
-
- Riess P, Zhang C, Saatman KE, Laurer HL, Longhi LG, Raghupathi R, Lenzlinger PM, Lifshitz J, Boockvar J, et al. (2002). Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery 51:1043–1052; discussion 1052–1054. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

