SERCA2a gene therapy in heart failure: an anti-arrhythmic positive inotrope
- PMID: 24138023
- PMCID: PMC3874695
- DOI: 10.1111/bph.12472
SERCA2a gene therapy in heart failure: an anti-arrhythmic positive inotrope
Abstract
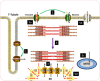
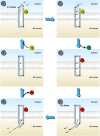
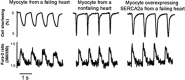
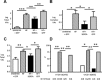
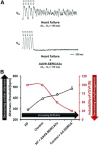
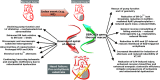
Therapeutic options that directly enhance cardiomyocyte contractility in chronic heart failure (HF) therapy are currently limited and do not improve prognosis. In fact, most positive inotropic agents, such as β-adrenoreceptor agonists and PDE inhibitors, which have been assessed in HF patients, cause increased mortality as a result of arrhythmia and sudden cardiac death. Cardiac sarcoplasmic reticulum Ca(2)(+) -ATPase2a (SERCA2a) is a key protein involved in sequestration of Ca(2)(+) into the sarcoplasmic reticulum (SR) during diastole. There is a reduction of SERCA2a protein level and function in HF, which has been successfully targeted via viral transfection of the SERCA2a gene into cardiac tissue in vivo. This has enhanced cardiac contractility and reduced mortality in several preclinical models of HF. Theoretical concerns have been raised regarding the possibility of arrhythmogenic adverse effects of SERCA2a gene therapy due to enhanced SR Ca(2)(+) load and induction of SR Ca(2)(+) leak as a result. Contrary to these concerns, SERCA2a gene therapy in a wide variety of preclinical models, including acute ischaemia/reperfusion, chronic pressure overload and chronic myocardial infarction, has resulted in a reduction in ventricular arrhythmias. The potential mechanisms for this unexpected beneficial effect, as well as mechanisms of enhancement of cardiac contractile function, are reviewed in this article.
Keywords: anti-arrhythmic; arrhythmia; gene therapy; heart failure.
© 2013 The British Pharmacological Society.
Figures
References
-
- Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. - PubMed
-
- Anderson ME, Braun AP, Wu Y, Lu T, Wu Y, Schulman H, et al. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharmacol Exp Ther. 1998;287:996–1006. - PubMed
-
- Andersson KB, Birkeland JAK, Finsen AV, Louch WE, Sjaastad I, Wang Y, et al. Moderate heart dysfunction in mice with inducible cardiomyocyte-specific excision of the Serca2 gene. J Mol Cell Cardiol. 2009;47:180–187. - PubMed
-
- Antipenko AY, Spielman AI, Kirchberger MA. Interactions of 6-gingerol and ellagic acid with the cardiac sarcoplasmic reticulum Ca2+-ATPase. J Pharmacol Exp Ther. 1999;290:227–234. - PubMed
-
- Aracena P, Tang W, Hamilton SL, Hidalgo C. Effects of S-glutathionylation and S-nitrosylation on calmodulin binding to triads and FKBP12 binding to type 1 calcium release channels. Antioxid Redox Signal. 2005;7:870–881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

