Uptake, coverage, and completion of quadrivalent human papillomavirus vaccine in the vaccine safety Datalink, July 2006-June 2011
- PMID: 24138765
- PMCID: PMC6708557
- DOI: 10.1016/j.jadohealth.2013.08.002
Uptake, coverage, and completion of quadrivalent human papillomavirus vaccine in the vaccine safety Datalink, July 2006-June 2011
Abstract
Purpose: The Advisory Committee on Immunization Practices recommended quadrivalent human papillomavirus vaccine (HPV4) for use in females in June 2006 and in males in October 2009. The objective of our study was to describe HPV4 uptake, single-dose coverage, and completion of the three-dose series among those 9-26 years of age, after the respective female and male vaccine licensures through June 2011.
Methods: The study population included members of eight managed care organizations participating in the Vaccine Safety Datalink; we abstracted demographic and comprehensive vaccine information from electronic health records.
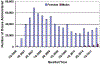
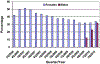
Results: We found one-dose coverage increasing throughout the study period, to a high of 37.7% among females and 1.3% among males in June 2011. Among those receiving at least one HPV4 dose, three-dose series completion was 42% for females and 30.2% for males.
Conclusions: Our results demonstrate low initiation and completion of the HPV4 series among those recommended to receive the vaccine. Although consistent with previous studies, these results highlight the continued need to develop, implement, and monitor strategies to increase HPV4 vaccine initiation and completion in younger adolescents to achieve maximum impact in reducing the burden of cervical cancer and other HPV-related diseases.
Keywords: Gardasil; HPV vaccines; Papillomavirus vaccines; Vaccination.
Copyright © 2013 Society for Adolescent Health and Medicine. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Subramanya D, Grivas PD. HPV and cervical cancer: Updates on an established relationship. Postgrad Med 2008;120:7–13. - PubMed
-
- American Cancer Society. Cancer facts & figures 2011. Atlanta, GA: American Cancer Society; 2011.
-
- Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011;60:1705–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical