Phosphorylated phospholamban stabilizes a compact conformation of the cardiac calcium-ATPase
- PMID: 24138857
- PMCID: PMC3797577
- DOI: 10.1016/j.bpj.2013.08.045
Phosphorylated phospholamban stabilizes a compact conformation of the cardiac calcium-ATPase
Abstract
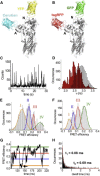
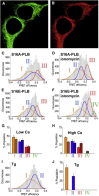
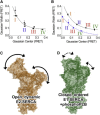
The sarcoendoplasmic reticulum calcium ATPase (SERCA) plays a key role in cardiac calcium handling and is considered a high-value target for the treatment of heart failure. SERCA undergoes conformational changes as it harnesses the chemical energy of ATP for active transport. X-ray crystallography has provided insight into SERCA structural substates, but it is not known how well these static snapshots describe in vivo conformational dynamics. The goals of this work were to quantify the direction and magnitude of SERCA motions as the pump performs work in live cardiac myocytes, and to identify structural determinants of SERCA regulation by phospholamban. We measured intramolecular fluorescence resonance energy transfer (FRET) between fluorescent proteins fused to SERCA cytoplasmic domains. We detected four discrete structural substates for SERCA expressed in cardiac muscle cells. The relative populations of these discrete states oscillated with electrical pacing. Low FRET states were most populated in low Ca (diastole), and were indicative of an open, disordered structure for SERCA in the E2 (Ca-free) enzymatic substate. High FRET states increased with Ca (systole), suggesting rigidly closed conformations for the E1 (Ca-bound) enzymatic substates. Notably, a special compact E1 state was observed after treatment with β-adrenergic agonist or with coexpression of phosphomimetic mutants of phospholamban. The data suggest that SERCA calcium binding induces the pump to undergo a transition from an open, dynamic conformation to a closed, ordered structure. Phosphorylated phospholamban stabilizes a unique conformation of SERCA that is characterized by a compact architecture.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
2-Color calcium pump reveals closure of the cytoplasmic headpiece with calcium binding.PLoS One. 2012;7(7):e40369. doi: 10.1371/journal.pone.0040369. Epub 2012 Jul 11. PLoS One. 2012. PMID: 22808146 Free PMC article.
-
Time-resolved FRET reveals the structural mechanism of SERCA-PLB regulation.Biochem Biophys Res Commun. 2014 Jun 27;449(2):196-201. doi: 10.1016/j.bbrc.2014.04.166. Epub 2014 May 9. Biochem Biophys Res Commun. 2014. PMID: 24813991 Free PMC article.
-
Conformational memory in the association of the transmembrane protein phospholamban with the sarcoplasmic reticulum calcium pump SERCA.J Biol Chem. 2017 Dec 29;292(52):21330-21339. doi: 10.1074/jbc.M117.794453. Epub 2017 Oct 29. J Biol Chem. 2017. PMID: 29081402 Free PMC article.
-
SR Ca(2+)-ATPase/phospholamban in cardiomyocyte function.J Card Fail. 1996 Dec;2(4 Suppl):S77-85. doi: 10.1016/s1071-9164(96)80062-5. J Card Fail. 1996. PMID: 8951564 Review.
-
The SarcoEndoplasmic Reticulum Calcium ATPase.Subcell Biochem. 2018;87:229-258. doi: 10.1007/978-981-10-7757-9_8. Subcell Biochem. 2018. PMID: 29464562 Review.
Cited by
-
A structural mechanism for calcium transporter headpiece closure.J Phys Chem B. 2015 Jan 29;119(4):1407-15. doi: 10.1021/jp511433v. Epub 2015 Jan 9. J Phys Chem B. 2015. PMID: 25531267 Free PMC article.
-
Redistribution of SERCA calcium pump conformers during intracellular calcium signaling.J Biol Chem. 2018 Jul 13;293(28):10843-10856. doi: 10.1074/jbc.RA118.002472. Epub 2018 May 15. J Biol Chem. 2018. PMID: 29764938 Free PMC article.
-
Phospholamban interactome in cardiac contractility and survival: A new vision of an old friend.J Mol Cell Cardiol. 2014 Dec;77:160-7. doi: 10.1016/j.yjmcc.2014.10.005. Epub 2014 Oct 18. J Mol Cell Cardiol. 2014. PMID: 25451386 Free PMC article. Review.
-
Preexisting domain motions underlie protonation-dependent structural transitions of the P-type Ca2+-ATPase.Phys Chem Chem Phys. 2017 Apr 12;19(15):10153-10162. doi: 10.1039/c7cp00243b. Phys Chem Chem Phys. 2017. PMID: 28374038 Free PMC article.
-
Development of Novel Intramolecular FRET-Based ABC Transporter Biosensors to Identify New Substrates and Modulators.Pharmaceutics. 2018 Oct 13;10(4):186. doi: 10.3390/pharmaceutics10040186. Pharmaceutics. 2018. PMID: 30322148 Free PMC article.
References
-
- Periasamy M., Huke S. SERCA pump level is a critical determinant of Ca2+ homeostasis and cardiac contractility. J. Mol. Cell. Cardiol. 2001;33:1053–1063. - PubMed
-
- Wold L.E., Dutta K., Davidoff A.J. Impaired SERCA function contributes to cardiomyocyte dysfunction in insulin resistant rats. J. Mol. Cell. Cardiol. 2005;39:297–307. - PubMed
-
- Schmitt J.P., Kamisago M., Seidman C.E. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003;299:1410–1413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

