The structural basis of cholesterol accessibility in membranes
- PMID: 24138860
- PMCID: PMC3797575
- DOI: 10.1016/j.bpj.2013.08.042
The structural basis of cholesterol accessibility in membranes
Abstract
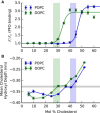
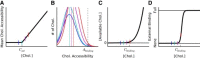
Although the majority of free cellular cholesterol is present in the plasma membrane, cholesterol homeostasis is principally regulated through sterol-sensing proteins that reside in the cholesterol-poor endoplasmic reticulum (ER). In response to acute cholesterol loading or depletion, there is rapid equilibration between the ER and plasma membrane cholesterol pools, suggesting a biophysical model in which the availability of plasma membrane cholesterol for trafficking to internal membranes modulates ER membrane behavior. Previous studies have predominantly examined cholesterol availability in terms of binding to extramembrane acceptors, but have provided limited insight into the structural changes underlying cholesterol activation. In this study, we use both molecular dynamics simulations and experimental membrane systems to examine the behavior of cholesterol in membrane bilayers. We find that cholesterol depth within the bilayer provides a reasonable structural metric for cholesterol availability and that this is correlated with cholesterol-acceptor binding. Further, the distribution of cholesterol availability in our simulations is continuous rather than divided into distinct available and unavailable pools. This data provide support for a revised cholesterol activation model in which activation is driven not by saturation of membrane-cholesterol interactions but rather by bulk membrane remodeling that reduces membrane-cholesterol affinity.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
25-Hydroxycholesterol increases the availability of cholesterol in phospholipid membranes.Biophys J. 2011 Feb 16;100(4):948-56. doi: 10.1016/j.bpj.2010.12.3728. Biophys J. 2011. PMID: 21320439 Free PMC article.
-
The importance of membrane defects-lessons from simulations.Acc Chem Res. 2014 Aug 19;47(8):2244-51. doi: 10.1021/ar4002729. Epub 2014 Jun 3. Acc Chem Res. 2014. PMID: 24892900
-
Lipid bilayer thickness determines cholesterol's location in model membranes.Soft Matter. 2016 Nov 28;12(47):9417-9428. doi: 10.1039/c6sm01777k. Soft Matter. 2016. PMID: 27801465
-
Molecular dynamics simulation studies of lipid bilayer systems.Acta Biochim Pol. 2000;47(3):601-11. Acta Biochim Pol. 2000. PMID: 11310963 Review.
-
Lipid-protein interplay and lateral organization in biomembranes.Chem Phys Lipids. 2015 Jul;189:48-55. doi: 10.1016/j.chemphyslip.2015.05.008. Epub 2015 May 30. Chem Phys Lipids. 2015. PMID: 26036778 Review.
Cited by
-
The signature of extracellular vesicles in hypoxic breast cancer and their therapeutic engineering.Cell Commun Signal. 2024 Oct 21;22(1):512. doi: 10.1186/s12964-024-01870-w. Cell Commun Signal. 2024. PMID: 39434182 Free PMC article. Review.
-
Cholesterol-Induced Nanoscale Variations in the Thickness of Phospholipid Membranes.Nano Lett. 2023 Mar 22;23(6):2421-2426. doi: 10.1021/acs.nanolett.2c04635. Epub 2023 Jan 27. Nano Lett. 2023. PMID: 36706024 Free PMC article.
-
A Coarse-Grained Molecular Dynamics Perspective on the Release of 5-Fluorouracil from Liposomes.Mol Pharm. 2024 Dec 2;21(12):6137-6152. doi: 10.1021/acs.molpharmaceut.4c00328. Epub 2024 Nov 8. Mol Pharm. 2024. PMID: 39515813 Free PMC article.
-
Oxysterols in Infectious Diseases.Adv Exp Med Biol. 2024;1440:125-147. doi: 10.1007/978-3-031-43883-7_7. Adv Exp Med Biol. 2024. PMID: 38036878
-
Visualization of accessible cholesterol using a GRAM domain-based biosensor.Nat Commun. 2023 Oct 25;14(1):6773. doi: 10.1038/s41467-023-42498-7. Nat Commun. 2023. PMID: 37880244 Free PMC article.
References
-
- Liscum L., Munn N.J. Intracellular cholesterol transport. Biochim. Biophys. Acta. 1999;1438:19–37. - PubMed
-
- Ohvo-Rekilä H., Ramstedt B., Slotte J.P. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 2002;41:66–97. - PubMed
-
- Purdy P.H., Fox M.H., Graham J.K. The fluidity of Chinese hamster ovary cell and bull sperm membranes after cholesterol addition. Cryobiology. 2005;51:102–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

