Endocannabinoids promote cocaine-induced impulsivity and its rapid dopaminergic correlates
- PMID: 24138924
- PMCID: PMC3943889
- DOI: 10.1016/j.biopsych.2013.09.005
Endocannabinoids promote cocaine-induced impulsivity and its rapid dopaminergic correlates
Abstract
Background: Impaired decision making, a hallmark of addiction, is hypothesized to arise from maladaptive plasticity in the mesolimbic dopamine pathway. The endocannabinoid system modulates dopamine activity through activation of cannabinoid type 1 receptors (CB1Rs). Here, we investigated whether impulsive behavior observed following cocaine exposure requires CB1R activation.
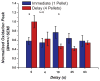
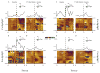
Methods: We trained rats in a delay-discounting task. Following acquisition of stable performance, rats were exposed to cocaine (10 mg/kg, intraperitoneal) every other day for 14 days and locomotor activity was measured. Two days later, delay-discounting performance was re-evaluated. To assess reversal of impulsivity, injections of a CB1R antagonist (1.5 mg/kg, intraperitoneal) or vehicle were given 30 minutes before the task. During the second experiment, aimed at preventing impulsivity rather than reversing it, CB1Rs were antagonized before each cocaine injection. In this experiment, subsecond dopamine release was measured in the nucleus accumbens during delay-discounting sessions before and after cocaine treatment.
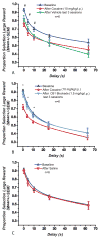
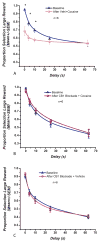
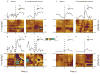
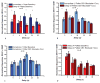
Results: Blockade of CB1Rs reversed and prevented cocaine-induced impulsivity. Electrochemical results showed that during baseline and following disruption of endocannabinoid signaling, there was a robust increase in dopamine for immediate large rewards compared with immediate small rewards, but this effect reversed when the delay for the large reward was 10 seconds. In contrast, dopamine release always increased for one-pellet options at minimal or moderate delays in vehicle-treated rats.
Conclusions: Endocannabinoids play a critical role in changes associated with cocaine exposure. Cannabinoid type 1 receptor blockade may thus counteract maladaptive alterations in afferents to dopamine neurons, thereby preventing changes in dopaminergic activity underlying a loss of self-control.
Keywords: CB1 receptors; cocaine; decision-making; dopamine; fast-scan cyclic voltammetry; self-control.
© 2013 Society of Biological Psychiatry Published by Society of Biological Psychiatry All rights reserved.
Conflict of interest statement
The authors report no biomedical financial interests or potential conflicts of interest.
Figures



Comment in
-
A discount on cannabinoids.Biol Psychiatry. 2014 Mar 15;75(6):432-4. doi: 10.1016/j.biopsych.2014.01.010. Epub 2014 Jan 27. Biol Psychiatry. 2014. PMID: 24560432 No abstract available.
Similar articles
-
Cocaine-induced endocannabinoid release modulates behavioral and neurochemical sensitization in mice.Addict Biol. 2015 Jan;20(1):91-103. doi: 10.1111/adb.12080. Epub 2013 Aug 4. Addict Biol. 2015. PMID: 23910902 Free PMC article.
-
Methamphetamine-induced dopamine terminal deficits in the nucleus accumbens are exacerbated by reward-associated cues and attenuated by CB1 receptor antagonism.Neuropharmacology. 2012 Jun;62(7):2192-201. doi: 10.1016/j.neuropharm.2012.01.013. Epub 2012 Jan 25. Neuropharmacology. 2012. PMID: 22306525 Free PMC article.
-
Endogenous dopamine and endocannabinoid signaling mediate cocaine-induced reversal of AMPAR synaptic potentiation in the nucleus accumbens shell.Neuropharmacology. 2018 Mar 15;131:154-165. doi: 10.1016/j.neuropharm.2017.12.011. Epub 2017 Dec 7. Neuropharmacology. 2018. PMID: 29225042 Free PMC article.
-
Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction.Neuropharmacology. 2005 Jun;48(8):1105-16. doi: 10.1016/j.neuropharm.2005.03.016. Neuropharmacology. 2005. PMID: 15878779 Review.
-
Drug addiction.Curr Top Behav Neurosci. 2009;1:309-46. doi: 10.1007/978-3-540-88955-7_13. Curr Top Behav Neurosci. 2009. PMID: 21104390 Free PMC article. Review.
Cited by
-
Discrepant effects of acute cocaine on impulsive choice (delay discounting) in female rats during an increasing- and adjusting-delay procedure.Psychopharmacology (Berl). 2015 Jul;232(14):2455-62. doi: 10.1007/s00213-015-3874-5. Epub 2015 Feb 28. Psychopharmacology (Berl). 2015. PMID: 25724278 Free PMC article.
-
Dissecting drug effects in preclinical models of impulsive choice: emphasis on glutamatergic compounds.Psychopharmacology (Berl). 2018 Mar;235(3):607-626. doi: 10.1007/s00213-017-4825-0. Epub 2018 Jan 6. Psychopharmacology (Berl). 2018. PMID: 29305628 Free PMC article. Review.
-
Effects of various cannabinoid ligands on choice behaviour in a rat model of gambling.Behav Pharmacol. 2016 Apr;27(2-3 Spec Issue):258-69. doi: 10.1097/FBP.0000000000000222. Behav Pharmacol. 2016. PMID: 26905189 Free PMC article.
-
Lateral habenula cannabinoid CB1 receptor involvement in drug-associated impulsive behavior.Neuropharmacology. 2021 Jul 1;192:108604. doi: 10.1016/j.neuropharm.2021.108604. Epub 2021 May 7. Neuropharmacology. 2021. PMID: 33965396 Free PMC article.
-
A transient dopamine signal encodes subjective value and causally influences demand in an economic context.Proc Natl Acad Sci U S A. 2017 Dec 26;114(52):E11303-E11312. doi: 10.1073/pnas.1706969114. Epub 2017 Nov 6. Proc Natl Acad Sci U S A. 2017. PMID: 29109253 Free PMC article.
References
-
- Ainslie G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychological bulletin. 1975;82:463–496. - PubMed
-
- Loguea W, Tobin H, Chelonis JJ, Wang RY, Geary N, Schachter S. Cocaine decreases self-control in rats: a preliminary report. Psychopharmacology. 1992;109:245–7. - PubMed
-
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. - PubMed
-
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

