RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention
- PMID: 24139042
- PMCID: PMC4098943
- DOI: 10.1016/j.neuron.2013.10.015
RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention
Erratum in
- Neuron. 2013 Nov 20;80(4):1102. Heusler, Aaron R [corrected to Haeusler, Aaron R]
Abstract
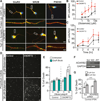
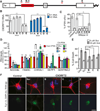
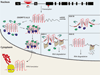
A hexanucleotide GGGGCC repeat expansion in the noncoding region of the C9ORF72 gene is the most common genetic abnormality in familial and sporadic amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). The function of the C9ORF72 protein is unknown, as is the mechanism by which the repeat expansion could cause disease. Induced pluripotent stem cell (iPSC)-differentiated neurons from C9ORF72 ALS patients revealed disease-specific (1) intranuclear GGGGCCexp RNA foci, (2) dysregulated gene expression, (3) sequestration of GGGGCCexp RNA binding protein ADARB2, and (4) susceptibility to excitotoxicity. These pathological and pathogenic characteristics were confirmed in ALS brain and were mitigated with antisense oligonucleotide (ASO) therapeutics to the C9ORF72 transcript or repeat expansion despite the presence of repeat-associated non-ATG translation (RAN) products. These data indicate a toxic RNA gain-of-function mechanism as a cause of C9ORF72 ALS and provide candidate antisense therapeutics and candidate human pharmacodynamic markers for therapy.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
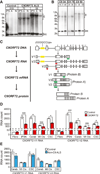
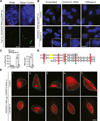
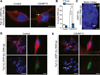

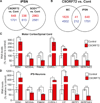
Comment in
-
C9orf72-associated FTD/ALS: when less is more.Neuron. 2013 Oct 16;80(2):257-8. doi: 10.1016/j.neuron.2013.10.010. Neuron. 2013. PMID: 24139028 Free PMC article.
References
-
- Ciura S, Lattante S, Le Ber I, Latouche M, Tostivint H, Brice A, Kabashi E. Loss of function of C9orf72 causes motor deficits in a zebrafish model of Amyotrophic Lateral Sclerosis. Ann. Neurol. 2013 May 30; - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

