Impaired hippocampal ripple-associated replay in a mouse model of schizophrenia
- PMID: 24139046
- PMCID: PMC3871857
- DOI: 10.1016/j.neuron.2013.09.014
Impaired hippocampal ripple-associated replay in a mouse model of schizophrenia
Abstract
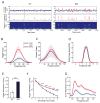
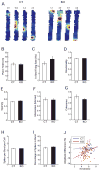
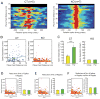
The cognitive symptoms of schizophrenia presumably result from impairments of information processing in neural circuits. We recorded neural activity in the hippocampus of freely behaving mice that had a forebrain-specific knockout of the synaptic plasticity-mediating phosphatase calcineurin and were previously shown to exhibit behavioral and cognitive abnormalities, recapitulating the symptoms of schizophrenia. Calcineurin knockout (KO) mice exhibited a 2.5-fold increase in the abundance of sharp-wave ripple (SWR) events during awake resting periods and single units in KO were overactive during SWR events. Pairwise measures of unit activity, however, revealed that the sequential reactivation of place cells during SWR events was completely abolished in KO. Since this relationship during postexperience awake rest periods has been implicated in learning, working memory, and subsequent memory consolidation, our findings provide a mechanism underlying impaired information processing that may contribute to the cognitive impairments in schizophrenia.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Of mice and men: what physiological correlates of cognitive deficits in a mouse model of schizophrenia tell us about psychiatric disease.Neuron. 2013 Oct 16;80(2):265-6. doi: 10.1016/j.neuron.2013.10.012. Neuron. 2013. PMID: 24139031
References
-
- Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron. 2006;52:179–196. - PubMed
-
- Behrens CJ, van den Boom LP, de Hoz L, Friedman A, Heinemann U. Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci. 2005;8:1560–1567. - PubMed
-
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. - PubMed
-
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. - PubMed
-
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

