Method to grow Actinobacillus pleuropneumoniae biofilm on a biotic surface
- PMID: 24139070
- PMCID: PMC4015846
- DOI: 10.1186/1746-6148-9-213
Method to grow Actinobacillus pleuropneumoniae biofilm on a biotic surface
Abstract
Background: Actinobacillus pleuropneumoniae is a Gram-negative bacterium and a member of the Pasteurellaceae family. This bacterium is the causative agent of porcine pleuropneumonia, which is a highly contagious respiratory disease causing important economical losses to the worldwide pig industry. It has been shown that A. pleuropneumoniae can form biofilms on abiotic surfaces (plastic and glass). Although in vitro models are extremely useful to gain information on biofilm formation, these models may not be representative of the conditions found at the mucosal surface of the host, which is the natural niche of A. pleuropneumoniae.
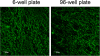
Results: In this paper, we describe a method to grow A. pleuropneumoniae biofilms on the SJPL cell line, which represents a biotic surface. A non-hemolytic, non-cytotoxic mutant of A. pleuropneumoniae was used in our assays and this allowed the SJPL cell monolayers to be exposed to A. pleuropneumoniae for longer periods. This resulted in the formation of biofilms on the cell monolayer after incubations of 24 and 48 h. The biofilms can be stained with fluorescent probes, such as a lectin against the polymer of N-acetyl-D-glucosamine present in the biofilm matrix, and easily observed by confocal laser scanning microscopy.
Conclusions: This is the first protocol that describes the formation of an A. pleuropneumoniae biofilm on a biotic surface. The advantage of this protocol is that it can be used to study biofilm formation in a context of host-pathogen interactions. The protocol could also be adapted to evaluate biofilm inhibitors or the efficacy of antibiotics in the presence of biofilms.
Figures
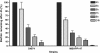


References
-
- Bossé JT, Janson H, Sheehan BJ, Beddek AJ, Rycroft AN, Kroll JS, Langford PR. Actinobacillus pleuropneumoniae: pathobiology and pathogenesis of infection. Microbes Infect. 2002;9:225–235. - PubMed
-
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;9:1318–1322. - PubMed
-
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;9:95–108. - PubMed
-
- Jacques M, Aragon V, Tremblay YDN. Biofilm formation in bacterial pathogens of veterinary importance. Anim Health Res Rev. 2010;9:97–121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

