Maternal adverse effects of different antenatal magnesium sulphate regimens for improving maternal and infant outcomes: a systematic review
- PMID: 24139447
- PMCID: PMC4015216
- DOI: 10.1186/1471-2393-13-195
Maternal adverse effects of different antenatal magnesium sulphate regimens for improving maternal and infant outcomes: a systematic review
Abstract
Background: Antenatal magnesium sulphate, widely used in obstetrics to improve maternal and infant outcomes, may be associated with adverse effects for the mother sufficient for treatment cessation. This systematic review aimed to quantify maternal adverse effects attributed to treatment, assess how adverse effects vary according to different regimens, and explore women's experiences with this treatment.
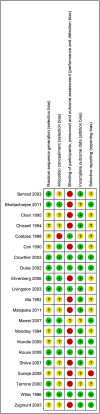
Methods: Bibliographic databases were searched from their inceptions to July 2012 for studies of any design that reported on maternal adverse effects associated with antenatal magnesium sulphate given to improve maternal or infant outcomes. Primary outcomes were life-threatening adverse effects of treatment (death, cardiac arrest, respiratory arrest). For randomised controlled trials, data were meta-analysed, and risk ratios (RR) pooled using fixed-effects or random-effects models. For non-randomised studies, data were tabulated by design, and presented as RR, odds ratios or percentages, and summarised narratively.
Results: A total of 143 publications were included (21 randomised trials, 15 non-randomised comparative studies, 32 case series and 75 reports of individual cases), of mixed methodological quality. Compared with placebo or no treatment, magnesium sulphate was not associated with an increased risk of maternal death, cardiac arrest or respiratory arrest. Magnesium sulphate significantly increased the risk of 'any adverse effects' overall (RR 4.62, 95% CI 2.42-8.83; 4 trials, 13,322 women), and treatment cessation due to adverse effects (RR 2.77; 95% CI 2.32-3.30; 5 trials, 13,666 women). Few subgroup differences were observed (between indications for use and treatment regimens). In one trial, a lower dose regimen (2 g/3 hours) compared with a higher dose regimen (5 g/4 hours) significantly reduced treatment cessation (RR 0.05; 95% CI 0.01-0.39, 126 women). Adverse effect estimates from studies of other designs largely supported data from randomised trials. Case reports supported an association between iatrogenic overdose of magnesium sulphate and life-threatening consequences.
Conclusions: Appropriate administration of antenatal magnesium sulphate was not shown to be associated with serious maternal adverse effects, though an increase in 'minor' adverse effects and treatment cessation was shown. Larger trials are needed to determine optimal regimens, achieving maximal effectiveness with minimal adverse effects, for each antenatal indication for use. Vigilance in the use of magnesium sulphate is essential for women's safety.
Figures





References
-
- Duley L, Henderson-Smart D, Chou D. Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database Syst Rev. 2010;13 CD000128. - PubMed
-
- Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst Rev. 2002;13 CD001060. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

