GPI-anchor and GPI-anchored protein expression in PMM2-CDG patients
- PMID: 24139637
- PMCID: PMC4016514
- DOI: 10.1186/1750-1172-8-170
GPI-anchor and GPI-anchored protein expression in PMM2-CDG patients
Abstract
Background: Mutations in PMM2 impair phosphomannomutase-2 activity and cause the most frequent congenital disorder of glycosylation, PMM2-CDG. Mannose-1-phosphate, that is deficient in this disorder, is also implicated in the biosynthesis of glycosylphosphatidyl inositol (GPI) anchors.
Objective: To evaluate whether GPI-anchor and GPI-anchored proteins are defective in PMM2-CDG patients.
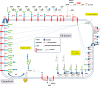
Methods: The expression of GPI-anchor and seven GPI-anchored proteins was evaluated by flow cytometry in different cell types from twelve PMM2-CDG patients. Additionally, neutrophil CD16 and plasma hepatic proteins were studied by Western blot. Transferrin glycoforms were evaluated by HPLC.
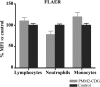
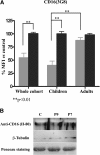
Results: Patients and controls had similar surface expression of GPI-anchor and most GPI-anchored proteins. Nevertheless, patients displayed a significantly diminished binding of two anti-CD16 antibodies (3G8 and KD1) to neutrophils and also of anti-CD14 (61D3) to monocytes. Interestingly, CD16 immunostaining and asialotransferrin levels significantly correlated with patients' age. Analysis by flow cytometry of CD14 with MΦP9, and CD16 expression in neutrophils by Western blot using H-80 ruled out deficiencies of these antigens.
Conclusions: PMM2 mutations do not impair GPI-anchor or GPI-anchored protein expression. However, the glycosylation anomalies caused by PMM2 mutations might affect the immunoreactivity of monoclonal antibodies and lead to incorrect conclusions about the expression of different proteins, including GPI-anchored proteins. Neutrophils and monocytes are sensitive to PMM2 mutations, leading to abnormal glycosylation in immune receptors, which might potentially affect their affinity to their ligands, and contribute to infection. This study also confirms less severe hypoglycosylation defects in older PMM2-CDG patients.
Figures
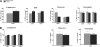


References
-
- Hennet T. Diseases of glycosylation beyond classical congenital disorders of glycosylation. Biochim Biophys Acta. 1820;8:1306–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

