Germline mutations in NFKB2 implicate the noncanonical NF-κB pathway in the pathogenesis of common variable immunodeficiency
- PMID: 24140114
- PMCID: PMC3824125
- DOI: 10.1016/j.ajhg.2013.09.009
Germline mutations in NFKB2 implicate the noncanonical NF-κB pathway in the pathogenesis of common variable immunodeficiency
Abstract
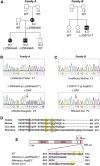
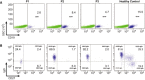
Common variable immunodeficiency (CVID) is a heterogeneous disorder characterized by antibody deficiency, poor humoral response to antigens, and recurrent infections. To investigate the molecular cause of CVID, we carried out exome sequence analysis of a family diagnosed with CVID and identified a heterozygous frameshift mutation, c.2564delA (p.Lys855Serfs(∗)7), in NFKB2 affecting the C terminus of NF-κB2 (also known as p100/p52 or p100/p49). Subsequent screening of NFKB2 in 33 unrelated CVID-affected individuals uncovered a second heterozygous nonsense mutation, c.2557C>T (p.Arg853(∗)), in one simplex case. Affected individuals in both families presented with an unusual combination of childhood-onset hypogammaglobulinemia with recurrent infections, autoimmune features, and adrenal insufficiency. NF-κB2 is the principal protein involved in the noncanonical NF-κB pathway, is evolutionarily conserved, and functions in peripheral lymphoid organ development, B cell development, and antibody production. In addition, Nfkb2 mouse models demonstrate a CVID-like phenotype with hypogammaglobulinemia and poor humoral response to antigens. Immunoblot analysis and immunofluorescence microscopy of transformed B cells from affected individuals show that the NFKB2 mutations affect phosphorylation and proteasomal processing of p100 and, ultimately, p52 nuclear translocation. These findings describe germline mutations in NFKB2 and establish the noncanonical NF-κB signaling pathway as a genetic etiology for this primary immunodeficiency syndrome.
Copyright © 2013 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Primary immunodeficiency diseases. Report of a WHO scientific group. Clin. Exp. Immunol. 1997;109(Suppl 1):1–28. - PubMed
-
- Oksenhendler E., Gérard L., Fieschi C., Malphettes M., Mouillot G., Jaussaud R., Viallard J.F., Gardembas M., Galicier L., Schleinitz N., DEFI Study Group Infections in 252 patients with common variable immunodeficiency. Clin. Infect. Dis. 2008;46:1547–1554. - PubMed
-
- Geha R.S., Notarangelo L.D., Casanova J.L., Chapel H., Conley M.E., Fischer A., Hammarström L., Nonoyama S., Ochs H.D., Puck J.M., International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee. J. Allergy Clin. Immunol. 2007;120:776–794. - PMC - PubMed
-
- Cunningham-Rundles C., Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin. Immunol. 1999;92:34–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

