Axitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trial
- PMID: 24140184
- PMCID: PMC4120767
- DOI: 10.1016/S1470-2045(13)70464-9
Axitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trial
Erratum in
- Lancet Oncol. 2014 Jun;15(7):e253
Abstract
Background: Population pharmacokinetic data suggest axitinib plasma exposure correlates with efficacy in metastatic renal-cell carcinoma. Axitinib dose titration might optimise exposure and improve outcomes. We prospectively assessed the efficacy and safety of axitinib dose titration in previously untreated patients with metastatic renal-cell carcinoma.
Methods: In this randomised, double-blind, multicentre, phase 2 study, patients were enrolled from 49 hospitals and outpatient clinics in the Czech Republic, Germany, Japan, Russia, Spain, and USA. Patients with treatment-naive metastatic renal-cell carcinoma received axitinib 5 mg twice daily during a 4 week lead-in period. Those patients with blood pressure 150/90 mm Hg or lower, no grade 3 or 4 treatment-related toxic effects, no dose reductions, and no more than two antihypertensive drugs for 2 consecutive weeks were stratified by Eastern Cooperative Oncology Group performance status (0 vs 1), and then randomly assigned (1:1) to either masked titration with axitinib to total twice daily doses of 7 mg, and then 10 mg, if tolerated, or placebo titration. Patients who did not meet these criteria continued without titration. The primary objective was comparison of the proportion of patients achieving an objective response between randomised groups. Safety analyses were based on all patients who received at least one dose of axitinib.
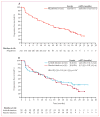
Findings: Between Sept 2, 2009, and Feb 28, 2011, we enrolled 213 patients, of whom 112 were randomly assigned to either the axitinib titration group (56 patients) or the placebo titration group (56 patients). 91 were not eligible for titration, and ten withdrew during the lead-in period. 30 patients (54%, 95% CI 40-67) in the axitinib titration group had an objective response, as did 19 patients (34%, 22-48]) in the placebo titration group (one-sided p=0·019). 54 (59%, 95% CI 49-70) of non-randomised patients achieved an objective response. Common grade 3 or worse, all-causality adverse events in treated patients were hypertension (ten [18%] of 56 in the axitinib titration group vs five [9%] of 56 in the placebo titration group vs 45 [49%] of 91 in the non-randomised group), diarrhoea (seven [13%] vs two [4%] vs eight [9%]), and decreased weight (four [7%] vs three [5%] vs six [7%]). One or more all-causality serious adverse events were reported in 15 (27%) patients in the axitinib titration group, 13 (23%) patients in the placebo titration group, and 35 (38%) non-randomised patients. The most common serious adverse events in all 213 patients were disease progression and dehydration (eight each [4%]), and diarrhoea, vomiting, pneumonia, and decreased appetite (four each [2%]).
Interpretation: The greater proportion of patients in the axitinib titration group achieving an objective response supports the concept of individual axitinib dose titration in selected patients with metastatic renal-cell carcinoma. Axitinib shows clinical activity with a manageable safety profile in treatment-naive patients with this disease.
Trial registration: ClinicalTrials.gov NCT00835978.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
BIR has served as an adviser for and received research funding from Pfizer. BM has served an as adviser for Roche and Astellas, and has received lecture fees from Roche, Novartis, Pfizer, and GlaxoSmithKline. TU has received lecture fees from Pfizer. VG has served as an adviser for and received lecture fees from Pfizer, Novartis, GlaxoSmithKline, Astellas, Bayer, and Roche. MNF has received research funding from Amgen, Altor Biosciences, Aveo, Bayer, Bristol-Myers Squibb, Eisai, Genentech, GlaxoSmithKline, and Pfizer, has received honoraria from Aveo, Altor Biosciences, Bayer, Genentech, GlaxoSmithKline, Prometheus, and Pfizer, and has served on speakers bureaus for Bayer, GlaxoSmithKline, Novartis, Pfizer, and Prometheus. JAA has served as an adviser for Pfizer. AHB, YKP, GIA, and DP are employees of and own stock in Pfizer. SK, employed at Pfizer at the time of the study described here, is currently employed by Mirna Therapeutics and owns stock in Pfizer and Mirna Therapeutics. EJ has served as an adviser for and received research funding from Pfizer.
Figures

Comment in
-
Axitinib dose titration: what's the limiting factor?Lancet Oncol. 2013 Nov;14(12):1152-4. doi: 10.1016/S1470-2045(13)70489-3. Epub 2013 Oct 18. Lancet Oncol. 2013. PMID: 24140182 No abstract available.
-
Kidney cancer: Axitinib destined for second place?Nat Rev Urol. 2013 Dec;10(12):678. doi: 10.1038/nrurol.2013.257. Epub 2013 Nov 12. Nat Rev Urol. 2013. PMID: 24217676 No abstract available.
References
-
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. - PubMed
-
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. - PubMed
-
- Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–11. - PubMed
-
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–68. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

