Contribution of endogenously produced reactive oxygen species to the activation of podocyte NLRP3 inflammasomes in hyperhomocysteinemia
- PMID: 24140862
- PMCID: PMC3945111
- DOI: 10.1016/j.freeradbiomed.2013.10.009
Contribution of endogenously produced reactive oxygen species to the activation of podocyte NLRP3 inflammasomes in hyperhomocysteinemia
Abstract
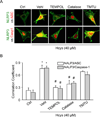
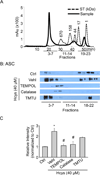
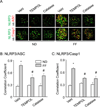
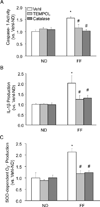
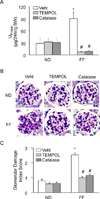
Hyperhomocysteinemia (hHcys) is an important pathogenic factor contributing to the progression of end-stage renal disease. Recent studies have demonstrated the implication of nicotinamide adenine dinucleotide phosphate oxidase-mediated NLRP3 inflammasome activation in the development of podocyte injury and glomerular sclerosis during hHcys. However, it remains unknown which reactive oxygen species (ROS) are responsible for this activation of NLRP3 inflammasomes and how such action of ROS is controlled. This study tested the contribution of common endogenous ROS including superoxide (O2(-)), hydrogen peroxide (H2O2), peroxynitrite (ONOO(-)), and hydroxyl radical (OH) to the activation of NLRP3 inflammasomes in mouse podocytes and glomeruli. In vitro, confocal microscopy and size-exclusion chromatography demonstrated that dismutation of O2(-) by 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (Tempol) and decomposition of H2O2 by catalase prevented Hcys-induced aggregation of NLRP3 inflammasome proteins and inhibited Hcys-induced caspase-1 activation and IL-1β production in mouse podocytes. However, scavenging of ONOO(-) or OH had no significant effect on either Hcys-induced NLRP3 inflammasome formation or activation. In vivo, scavenging of O2(-) by Tempol and removal of H2O2 by catalase substantially inhibited NLRP3 inflammasome formation and activation in glomeruli of hHcys mice as shown by reduced colocalization of NLRP3 with ASC or caspase-1 and inhibition of caspase-1 activation and IL-1β production. Furthermore, Tempol and catalase significantly attenuated hHcys-induced glomerular injury. In conclusion, endogenously produced O2(-) and H2O2 primarily contribute to NLRP3 inflammasome formation and activation in mouse glomeruli resulting in glomerular injury or consequent sclerosis during hHcys.
Keywords: Free radicals; Glomerular sclerosis; Homocysteine; NLRP3 inflammasome; Redox signaling.
© 2013 Elsevier Inc. All rights reserved.
Figures


References
-
- Perla-Kajan J, Jakubowski H. Paraoxonase 1 and homocysteine metabolism. Amino Acids. 2012;43:1405–1417. - PubMed
-
- Cavalca V, Cighetti G, Bamonti F, Loaldi A, Bortone L, Novembrino C, De Franceschi M, Belardinelli R, Guazzi MD. Oxidative stress and homocysteine in coronary artery disease. Clin Chem. 2001;47:887–892. - PubMed
-
- Bialecka M, Kurzawski M, Roszmann A, Robowski P, Sitek EJ, Honczarenko K, Gorzkowska A, Budrewicz S, Mak M, Jarosz M, Golab-Janowska M, Koziorowska-Gawron E, Drozdzik M, Slawek J. Association of COMT, MTHFR, and SLC19A1(RFC-1) polymorphisms with homocysteine blood levels and cognitive impairment in Parkinson's disease. Pharmacogenet Genomics. 2012;22:716–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

