Optogenetic examination identifies a context-specific role for orexins/hypocretins in anxiety-related behavior
- PMID: 24140988
- PMCID: PMC4155939
- DOI: 10.1016/j.physbeh.2013.10.005
Optogenetic examination identifies a context-specific role for orexins/hypocretins in anxiety-related behavior
Abstract
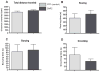
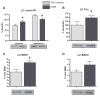
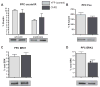
Maladaptation to stress is associated with psychopathology. However, our understanding of the underlying neural circuitry involved in adaptations to stress is limited. Previous work from our lab indicated the paraventricular hypothalamic neuropeptides orexins/hypocretins regulate behavioral and neuroendocrine responses to stress. To further elucidate the role of orexins in adaptation to stress, we employed optogenetic techniques to specifically examine the effects of orexin cell activation on behavior in the social interaction test and in the home cage as well as orexin receptor 1 internalization and ERK phosphorylation in brain regions receiving orexin inputs. In the social interaction test, optogenetic stimulation of orexin neurons decreased time spent in the interaction zone while increasing the frequency of entries into the interaction zone. In addition, optogenetic stimulation of orexin neurons increased the total distance traveled in the social interaction arena but had no effect on their home cage behavior. Together, these results suggest that orexin release increases anxiety in the social interaction test while increasing the salience of novel but not familiar environmental stimuli. Consistent with activation of orexin neurons, optogenetic stimulation increased orexin receptor1 internalization and ERK phosphorylation in the paraventricular thalamus (PVT) and locus coeruleus (LC), two regions heavily innervated by orexin neurons. Together these results show for the first time that elevation of orexin activity, possibly in the PVT and LC, is associated with increased anxiety, activity, and arousal in a context-specific manner.
Keywords: Anxiety; Hypocretins; Optogenetics; Orexins; Social interaction; Stress.
© 2013.
Figures



Similar articles
-
Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats.Psychopharmacology (Berl). 2010 Oct;212(2):251-65. doi: 10.1007/s00213-010-1948-y. Epub 2010 Jul 20. Psychopharmacology (Berl). 2010. PMID: 20645079
-
Orexin signaling during social defeat stress influences subsequent social interaction behaviour and recognition memory.Behav Brain Res. 2019 Jan 1;356:444-452. doi: 10.1016/j.bbr.2018.05.032. Epub 2018 Jun 11. Behav Brain Res. 2019. PMID: 29902478
-
Activation of orexin/hypocretin projections to basal forebrain and paraventricular thalamus by acute nicotine.Brain Res Bull. 2008 Dec 16;77(6):367-73. doi: 10.1016/j.brainresbull.2008.09.014. Epub 2008 Oct 23. Brain Res Bull. 2008. PMID: 18950690 Free PMC article.
-
Optogenetic control of hypocretin (orexin) neurons and arousal circuits.Curr Top Behav Neurosci. 2015;25:367-78. doi: 10.1007/7854_2014_364. Curr Top Behav Neurosci. 2015. PMID: 25502546 Free PMC article. Review.
-
Control of arousal by the orexin neurons.Curr Opin Neurobiol. 2013 Oct;23(5):752-9. doi: 10.1016/j.conb.2013.04.008. Epub 2013 May 15. Curr Opin Neurobiol. 2013. PMID: 23683477 Free PMC article. Review.
Cited by
-
Inhibitory Interplay between Orexin Neurons and Eating.Curr Biol. 2016 Sep 26;26(18):2486-2491. doi: 10.1016/j.cub.2016.07.013. Epub 2016 Aug 18. Curr Biol. 2016. PMID: 27546579 Free PMC article.
-
The Central Nervous Mechanism of Stress-Promoting Cancer Progression.Int J Mol Sci. 2022 Oct 21;23(20):12653. doi: 10.3390/ijms232012653. Int J Mol Sci. 2022. PMID: 36293510 Free PMC article. Review.
-
Reduced Orexin System Function Contributes to Resilience to Repeated Social Stress.eNeuro. 2018 Apr 16;5(2):ENEURO.0273-17.2018. doi: 10.1523/ENEURO.0273-17.2018. eCollection 2018 Mar-Apr. eNeuro. 2018. PMID: 29662948 Free PMC article.
-
Exploring the role of orexins in the modulation of social reward.Psychopharmacology (Berl). 2025 Feb;242(2):401-412. doi: 10.1007/s00213-024-06688-5. Epub 2024 Sep 20. Psychopharmacology (Berl). 2025. PMID: 39302438 Free PMC article.
-
Sex- and Age-dependent Effects of Orexin 1 Receptor Blockade on Open-Field Behavior and Neuronal Activity.Neuroscience. 2018 Jun 15;381:11-21. doi: 10.1016/j.neuroscience.2018.04.005. Epub 2018 Apr 18. Neuroscience. 2018. PMID: 29678754 Free PMC article.
References
-
- Castro MG, Morrison E, Perone MJ, Brown OA, Murray CA, Ahmed I, et al. Corticotropin-releasing hormone receptor type 1: generation and characterization of polyclonal antipeptide antibodies and their localization in pituitary cells and cortical neurones in vitro. J Neuroendocrinol. 1996;8:521–31. - PubMed
-
- Chang H, Saito T, Ohiwa N, Tateoka M, Deocaris CC, Fujikawa T, et al. Inhibitory effects of an orexin-2 receptor antagonist on orexin A- and stress-induced ACTH responses in conscious rats. Neurosci Res. 2007;57:462–6. - PubMed
-
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

