Minor antigen distribution predicts site-specific graft-versus-tumor activity of adoptively transferred, minor antigen-specific CD8 T Cells
- PMID: 24141010
- PMCID: PMC3997266
- DOI: 10.1016/j.bbmt.2013.10.009
Minor antigen distribution predicts site-specific graft-versus-tumor activity of adoptively transferred, minor antigen-specific CD8 T Cells
Abstract
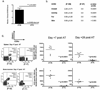
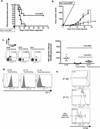
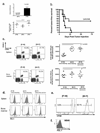
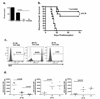
The clinical success of allogeneic T cell therapy for cancer relies on the selection of antigens that can effectively elicit antitumor responses with minimal toxicity toward nonmalignant tissues. Although minor histocompatibility antigens (MiHA) represent promising targets, broad expression of these antigens has been associated with poor responses and T cell dysfunction that may not be prevented by targeting MiHA with limited expression. In this study, we hypothesized that antitumor activity of MiHA-specific CD8 T cells after allogeneic bone marrow transplantation (BMT) is determined by the distribution of antigen relative to the site of tumor growth. To test this hypothesis, we utilized the clinically relevant male-specific antigen HY and studied the fate of adoptively transferred, HY-CD8(+) T cells (HY-CD8) against a HY-expressing epithelial tumor (MB49) and pre-B cell leukemia (HY-E2APBX ALL) in BMT recipients. Transplants were designed to produce broad HY expression in nonhematopoietic tissues (female → male BMT, [F → M]), restricted HY expression in hematopoietic tissues (male → female BMT, [M → F]) tissues, and no HY tissue expression (female → female BMT, [F → F]). Broad HY expression induced poor responses to MB49 despite sublethal graft-versus-host disease and accumulation of HY-CD8 in secondary lymphoid tissues. Antileukemia responses, however, were preserved. In contrast, restriction of HY expression to hematopoietic tissues restored MB49 responses but resulted in a loss of antileukemia responses. We concluded that target alloantigen expression in the same compartment of tumor growth impairs CD8 responses to both solid and hematologic tumors.
Keywords: Acute lymphoblastic leukemia; Adoptive T cell therapy; Allogeneic transplantation; CD8 T cell function; Minor histocompatibility antigens.
Copyright © 2014 American Society for Blood and Marrow Transplantation. All rights reserved.
Conflict of interest statement
Financial Disclosure Statement: The authors have no financial conflicts of interest to declare.
Figures


Similar articles
-
Graft-versus-host disease impairs vaccine responses through decreased CD4+ and CD8+ T cell proliferation and increased perforin-mediated CD8+ T cell apoptosis.J Immunol. 2013 Feb 1;190(3):1351-9. doi: 10.4049/jimmunol.1200391. Epub 2012 Dec 28. J Immunol. 2013. PMID: 23275602 Free PMC article.
-
HY-Specific Induced Regulatory T Cells Display High Specificity and Efficacy in the Prevention of Acute Graft-versus-Host Disease.J Immunol. 2015 Jul 15;195(2):717-25. doi: 10.4049/jimmunol.1401250. Epub 2015 Jun 5. J Immunol. 2015. PMID: 26048147 Free PMC article.
-
Donor antigen-presenting cells regulate T-cell expansion and antitumor activity after allogeneic bone marrow transplantation.Biol Blood Marrow Transplant. 2004 Aug;10(8):540-51. doi: 10.1016/j.bbmt.2004.05.007. Biol Blood Marrow Transplant. 2004. PMID: 15282532
-
Strategies for the identification of T cell-recognized tumor antigens in hematological malignancies for improved graft-versus-tumor responses after allogeneic blood and marrow transplantation.Biol Blood Marrow Transplant. 2015 Jun;21(6):1000-7. doi: 10.1016/j.bbmt.2014.11.001. Epub 2014 Nov 20. Biol Blood Marrow Transplant. 2015. PMID: 25459643 Free PMC article. Review.
-
Minor histocompatibility antigens--targets of graft versus leukemia responses.Int J Hematol. 2002 Aug;76 Suppl 2:155-61. doi: 10.1007/BF03165108. Int J Hematol. 2002. PMID: 12430918 Review.
Cited by
-
Defining novel parameters for the optimal priming and expansion of minor histocompatibility antigen-specific T cells in culture.J Transl Med. 2015 Apr 19;13:123. doi: 10.1186/s12967-015-0495-z. J Transl Med. 2015. PMID: 25925868 Free PMC article.
-
TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance.Sci Transl Med. 2017 Nov 22;9(417):eaag1209. doi: 10.1126/scitranslmed.aag1209. Sci Transl Med. 2017. PMID: 29167392 Free PMC article.
-
Escape from thymic deletion and anti-leukemic effects of T cells specific for hematopoietic cell-restricted antigen.Nat Commun. 2018 Jan 15;9(1):225. doi: 10.1038/s41467-017-02665-z. Nat Commun. 2018. PMID: 29335408 Free PMC article.
-
Challenges and opportunities of allogeneic donor-derived CAR T cells.Curr Opin Hematol. 2015 Nov;22(6):509-515. doi: 10.1097/MOH.0000000000000181. Curr Opin Hematol. 2015. PMID: 26390167 Free PMC article. Review.
-
CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity.Nat Commun. 2016 Jul 27;7:12320. doi: 10.1038/ncomms12320. Nat Commun. 2016. PMID: 27460500 Free PMC article.
References
-
- Miller JS, Warren EH, van den Brink MR, et al. NCI First International Workshop on the Biology, Prevention and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on the Biology Underlying Recurrence of Disease following Allogeneic HSCT. Biol Blood Marrow Transplant. 2010;16(5):565–586. - PMC - PubMed
-
- Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–4383. - PubMed
-
- Matte-Martone C, Venkatesan S, Tan HS, et al. Graft-versus-leukemia (GVL) against mouse blast-crisis chronic myelogenous leukemia (BCML) and chronic-phase chronic myelogenous leukemia (CP-CML): shared mechanisms of T cell killing but programmed death ligands render CP-CML, and not BC-CML GVL resistant. J Immunol. 2011;15:1653–1663. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

