Crude and purified proteasome activity assays are affected by type of microplate
- PMID: 24141075
- PMCID: PMC3901411
- DOI: 10.1016/j.ab.2013.10.018
Crude and purified proteasome activity assays are affected by type of microplate
Abstract
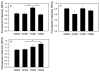
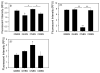
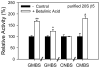
Measurement of proteasome activity is fast becoming a commonly used assay in many laboratories. The most common method to measure proteasome activity involves measuring the release of fluorescent tags from peptide substrates in black microplates. Comparisons of black plates used for measuring fluorescence with different properties show that the microplate properties significantly affect the measured activities of the proteasome. The microplate that gave the highest reading of trypsin-like activity of the purified 20S proteasome gave the lowest reading of chymotrypsin-like activity of the 20S proteasome. Plates with medium binding surfaces from two different companies showed an approximately 2-fold difference in caspase-like activity for purified 20S proteasomes. Even standard curves generated using free 7-amino-4-methylcoumarin (AMC) were affected by the microplate used. As such, significantly different proteasome activities, as measured in nmol AMC released/mg/min, were obtained for purified 20S proteasomes as well as crude heart and liver samples when using different microplates. The naturally occurring molecule betulinic acid activated the chymotrypsin-like proteasome activity in three different plates but did not affect the proteasome activity in the nonbinding surface microplate. These findings suggest that the type of proteasome activity being measured and sample type are important when selecting a microplate.
Keywords: Betulinic acid; Microplate; Proteasome; Proteolytic activity.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
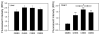




References
-
- Gomes AV, Zong C, Edmondson RD, Li X, Stefani E, Zhang J, Jones RC, Thyparambil S, Wang GW, Qiao X, Bardag-Gorce F, Ping P. Mapping the murine cardiac 26S proteasome complexes. Circ Res. 2006;99:362–71. - PubMed
-
- Gomes AV, Zong C, Ping P. Protein degradation by the 26S proteasome system in the normal and stressed myocardium. Antioxid Redox Signal. 2006;8:1677–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

