The neurobiology of alcohol consumption and alcoholism: an integrative history
- PMID: 24141171
- PMCID: PMC3867277
- DOI: 10.1016/j.pbb.2013.10.009
The neurobiology of alcohol consumption and alcoholism: an integrative history
Abstract
Studies of the neurobiological predisposition to consume alcohol (ethanol) and to transition to uncontrolled drinking behavior (alcoholism), as well as studies of the effects of alcohol on brain function, started a logarithmic growth phase after the repeal of the 18th Amendment to the United States Constitution. Although the early studies were primitive by current technological standards, they clearly demonstrated the effects of alcohol on brain structure and function, and by the end of the 20th century left little doubt that alcoholism is a "disease" of the brain. This review traces the history of developments in the understanding of ethanol's effects on the most prominent inhibitory and excitatory systems of brain (GABA and glutamate neurotransmission). This neurobiological information is integrated with knowledge of ethanol's actions on other neurotransmitter systems to produce an anatomical and functional map of ethanol's properties. Our intent is limited in scope, but is meant to provide context and integration of the actions of ethanol on the major neurobiologic systems which produce reinforcement for alcohol consumption and changes in brain chemistry that lead to addiction. The developmental history of neurobehavioral theories of the transition from alcohol drinking to alcohol addiction is presented and juxtaposed to the neurobiological findings. Depending on one's point of view, we may, at this point in history, know more, or less, than we think we know about the neurobiology of alcoholism.
Keywords: Addiction; Alcohol research history; GABA; Glutamate; Mesolimbic dopamine system; Reward.
© 2013.
Figures
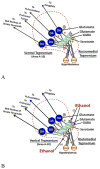
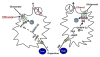
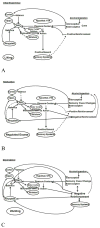
References
-
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Bedogni G, Caputo F, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46:312–7. - PubMed
-
- Aguayo LG. Ethanol potentiates the GABAA-activated Cl-current in mouse hippocampal and cortical neurons. Eur J Pharmacol. 1990;187:127–30. - PubMed
-
- Allan AM, Harris RA. Acute and chronic ethanol treatments alter GABA receptor-operated chloride channels. Pharmacol Biochem Behav. 1987;27:665–70. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

