Age-associated defects in EphA2 signaling impair the migration of human cardiac progenitor cells
- PMID: 24141256
- PMCID: PMC3888551
- DOI: 10.1161/CIRCULATIONAHA.113.004698
Age-associated defects in EphA2 signaling impair the migration of human cardiac progenitor cells
Erratum in
-
Correction.Circulation. 2015 Jul 28;132(4):e30. doi: 10.1161/CIR.0000000000000187. Circulation. 2015. PMID: 26216092 No abstract available.
-
Correction.Circulation. 2015 Dec 1;132(22):e361. doi: 10.1161/CIR.0000000000000339. Circulation. 2015. PMID: 26621664 No abstract available.
Retraction in
-
Retraction of: Age-Associated Defects in EphA2 Signaling Impair the Migration of Human Cardiac Progenitor Cells.Circulation. 2019 Feb 12;139(7):e39. doi: 10.1161/CIR.0000000000000643. Circulation. 2019. PMID: 30586778 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern.Circ Res. 2019 Jan 18;124(2):e4-e5. doi: 10.1161/RES.0000000000000241. Circ Res. 2019. PMID: 30582460 No abstract available.
-
Expression of Concern.Circulation. 2019 Jan 15;139(3):e5-e6. doi: 10.1161/CIR.0000000000000639. Circulation. 2019. PMID: 30615475 No abstract available.
Abstract
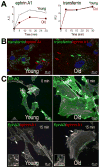
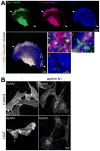
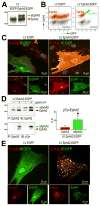
Background: Aging negatively impacts on the function of resident human cardiac progenitor cells (hCPCs). Effective regeneration of the injured heart requires mobilization of hCPCs to the sites of damage. In the young heart, signaling by the guidance receptor EphA2 in response to the ephrin A1 ligand promotes hCPC motility and improves cardiac recovery after infarction.
Methods and results: We report that old hCPCs are characterized by cell-autonomous inhibition of their migratory ability ex vivo and impaired translocation in vivo in the damaged heart. EphA2 expression was not decreased in old hCPCs; however, the elevated level of reactive oxygen species in aged cells induced post-translational modifications of the EphA2 protein. EphA2 oxidation interfered with ephrin A1-stimulated receptor auto-phosphorylation, activation of Src family kinases, and caveolin-1-mediated internalization of the receptor. Cellular aging altered the EphA2 endocytic route, affecting the maturation of EphA2-containing endosomes and causing premature signal termination. Overexpression of functionally intact EphA2 in old hCPCs corrected the defects in endocytosis and downstream signaling, enhancing cell motility. Based on the ability of phenotypically young hCPCs to respond efficiently to ephrin A1, we developed a novel methodology for the prospective isolation of live hCPCs with preserved migratory capacity and growth reserve.
Conclusions: Our data demonstrate that the ephrin A1/EphA2 pathway may serve as a target to facilitate trafficking of hCPCs in the senescent myocardium. Importantly, EphA2 receptor function can be implemented for the selection of hCPCs with high therapeutic potential, a clinically relevant strategy that does not require genetic manipulation of stem cells.
Keywords: aging; cell movement; receptor, EphA2; stem cells.
Conflict of interest statement
Figures











Comment in
-
Heart factory or fiction?: cardiac progenitor cells and regeneration.Circulation. 2013 Nov 12;128(20):2181-2. doi: 10.1161/CIRCULATIONAHA.113.006262. Epub 2013 Oct 18. Circulation. 2013. PMID: 24141257 No abstract available.
References
-
- Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. - PubMed
-
- Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Muller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. - PubMed
-
- Kajstura J, Gurusamy N, Ogorek B, Goichberg P, Clavo-Rondon C, Hosoda T, D’Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P. Myocyte turnover in the aging human heart. Circ Res. 2010;107:1374–1386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG017042/AG/NIA NIH HHS/United States
- R01 HL111183/HL/NHLBI NIH HHS/United States
- P01 AG043353/AG/NIA NIH HHS/United States
- R01 HL114346/HL/NHLBI NIH HHS/United States
- P01 HL092868/HL/NHLBI NIH HHS/United States
- R01 HL091021/HL/NHLBI NIH HHS/United States
- R01 HL039902/HL/NHLBI NIH HHS/United States
- R01 HL105532/HL/NHLBI NIH HHS/United States
- R01 AG037495/AG/NIA NIH HHS/United States
- R01 AG026107/AG/NIA NIH HHS/United States
- R01 HL065577/HL/NHLBI NIH HHS/United States
- R37 HL081737/HL/NHLBI NIH HHS/United States
- R01 AG037490/AG/NIA NIH HHS/United States
- P01 HL078825/HL/NHLBI NIH HHS/United States
- P01 AG023071/AG/NIA NIH HHS/United States
- R01 HL065573/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

