Common medial frontal mechanisms of adaptive control in humans and rodents
- PMID: 24141310
- PMCID: PMC3840072
- DOI: 10.1038/nn.3549
Common medial frontal mechanisms of adaptive control in humans and rodents
Abstract
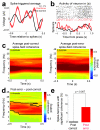
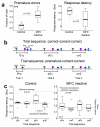
In this report we describe how common brain networks within the medial frontal cortex (MFC) facilitate adaptive behavioral control in rodents and humans. We demonstrate that after errors, low-frequency oscillations below 12 Hz are modulated over the midfrontal cortex in humans and within the prelimbic and anterior cingulate regions of the MFC in rats. These oscillations were phase locked between the MFC and motor areas in both rats and humans. In rats, single neurons that encoded prior behavioral outcomes were phase coherent with low-frequency field oscillations, particularly after errors. Inactivating the medial frontal regions in rats led to impaired behavioral adjustments after errors, eliminated the differential expression of low-frequency oscillations after errors and increased low-frequency spike-field coupling within the motor cortex. Our results describe a new mechanism for behavioral adaptation through low-frequency oscillations and elucidate how medial frontal networks synchronize brain activity to guide performance.
Figures
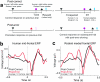
 - tone; ▲ - release, and
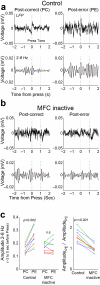
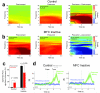
- tone; ▲ - release, and  – reward. Imperative tones occurred at the target time on 50% of trials. b) Average event-related potentials over mid-frontal cortex (electrode Cz) in humans aligned to the target time. Amplitudes were significantly increased on post-error (red) vs. post-correct (black) trials. c) Rodent medial frontal field potentials were also significantly increased on post-error (red) vs. post-correct (black) trials, and highly similar to humans. Data is from 28 medial frontal channels in 5 rats and is aligned to the target time.
– reward. Imperative tones occurred at the target time on 50% of trials. b) Average event-related potentials over mid-frontal cortex (electrode Cz) in humans aligned to the target time. Amplitudes were significantly increased on post-error (red) vs. post-correct (black) trials. c) Rodent medial frontal field potentials were also significantly increased on post-error (red) vs. post-correct (black) trials, and highly similar to humans. Data is from 28 medial frontal channels in 5 rats and is aligned to the target time.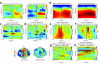

Comment in
-
Oh, rats! Post-error behavioral adjustment in creatures great and small.Nat Neurosci. 2013 Dec;16(12):1715-6. doi: 10.1038/nn.3577. Nat Neurosci. 2013. PMID: 24270271 No abstract available.
References
-
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The Role of the Medial Frontal Cortex in Cognitive Control. Science. 2004;306:443–447. - PubMed
-
- Holroyd CB, Coles MGH. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. - PubMed
-
- Rushworth MFS, Behrens TEJ. Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience. 2008;11:389–397. - PubMed
-
- vanMeel CS, Heslenfeld DJ, Oosterlaan J, Sergeant JA. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): the role of error processing. Psychiatry Res. 2007;151:211–220. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

