Integration of the olfactory code across dendritic claws of single mushroom body neurons
- PMID: 24141312
- PMCID: PMC3908930
- DOI: 10.1038/nn.3547
Integration of the olfactory code across dendritic claws of single mushroom body neurons
Abstract
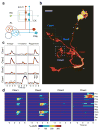
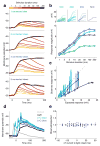
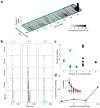
In the olfactory system, sensory inputs are arranged in different glomerular channels, which respond in combinatorial ensembles to the various chemical features of an odor. We investigated where and how this combinatorial code is read out deeper in the brain. We exploited the unique morphology of neurons in the Drosophila mushroom body, which receive input on large dendritic claws. Imaging odor responses of these dendritic claws revealed that input channels with distinct odor tuning converge on individual mushroom body neurons. We determined how these inputs interact to drive the cell to spike threshold using intracellular recordings to examine mushroom body responses to optogenetically controlled input. Our results provide an elegant explanation for the characteristic selectivity of mushroom body neurons: these cells receive different types of input and require those inputs to be coactive to spike. These results establish the mushroom body as an important site of integration in the fly olfactory system.
Conflict of interest statement
Figures


Similar articles
-
Presynaptic developmental plasticity allows robust sparse wiring of the Drosophila mushroom body.Elife. 2020 Jan 8;9:e52278. doi: 10.7554/eLife.52278. Elife. 2020. PMID: 31913123 Free PMC article.
-
Olfactory representations by Drosophila mushroom body neurons.J Neurophysiol. 2008 Feb;99(2):734-46. doi: 10.1152/jn.01283.2007. Epub 2007 Dec 19. J Neurophysiol. 2008. PMID: 18094099
-
Separate But Interactive Parallel Olfactory Processing Streams Governed by Different Types of GABAergic Feedback Neurons in the Mushroom Body of a Basal Insect.J Neurosci. 2019 Oct 30;39(44):8690-8704. doi: 10.1523/JNEUROSCI.0088-19.2019. Epub 2019 Sep 23. J Neurosci. 2019. PMID: 31548236 Free PMC article.
-
The olfactory bulb: coding and processing of odor molecule information.Science. 1999 Oct 22;286(5440):711-5. doi: 10.1126/science.286.5440.711. Science. 1999. PMID: 10531048 Review.
-
Mushroom body memoir: from maps to models.Nat Rev Neurosci. 2003 Apr;4(4):266-75. doi: 10.1038/nrn1074. Nat Rev Neurosci. 2003. PMID: 12671643 Review. No abstract available.
Cited by
-
Neural Circuits Underlying Behavioral Flexibility: Insights From Drosophila.Front Behav Neurosci. 2022 Jan 6;15:821680. doi: 10.3389/fnbeh.2021.821680. eCollection 2021. Front Behav Neurosci. 2022. PMID: 35069145 Free PMC article. Review.
-
Evolution of neural circuitry and cognition.Biol Lett. 2024 May;20(5):20230576. doi: 10.1098/rsbl.2023.0576. Epub 2024 May 15. Biol Lett. 2024. PMID: 38747685 Free PMC article. Review.
-
The connectome of the adult Drosophila mushroom body provides insights into function.Elife. 2020 Dec 14;9:e62576. doi: 10.7554/eLife.62576. Elife. 2020. PMID: 33315010 Free PMC article.
-
The neuronal architecture of the mushroom body provides a logic for associative learning.Elife. 2014 Dec 23;3:e04577. doi: 10.7554/eLife.04577. Elife. 2014. PMID: 25535793 Free PMC article.
-
Behavior Reveals Selective Summation and Max Pooling among Olfactory Processing Channels.Neuron. 2016 Jul 20;91(2):425-38. doi: 10.1016/j.neuron.2016.06.011. Epub 2016 Jun 30. Neuron. 2016. PMID: 27373835 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

