The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus
- PMID: 24141387
- PMCID: PMC3883080
- DOI: 10.1038/ni.2741
The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus
Abstract
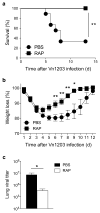
Highly pathogenic avian influenza viruses pose a continuing global threat. Current vaccines will not protect against newly evolved pandemic viruses. The creation of 'universal' vaccines has been unsuccessful because the immunological mechanisms that promote heterosubtypic immunity are incompletely defined. We found here that rapamycin, an immunosuppressive drug that inhibits the kinase mTOR, promoted cross-strain protection against lethal infection with influenza virus of various subtypes when administered during immunization with influenza virus subtype H3N2. Rapamycin reduced the formation of germinal centers and inhibited class switching in B cells, which yielded a unique repertoire of antibodies that mediated heterosubtypic protection. Our data established a requirement for the mTORC1 complex in B cell class switching and demonstrated that rapamycin skewed the antibody response away from high-affinity variant epitopes and targeted more conserved elements of hemagglutinin. Our findings have implications for the design of a vaccine against influenza virus.
Figures





Comment in
-
Infectious disease: hushing mTOR boosts immunity to pathogens.Nat Rev Immunol. 2013 Dec;13(12):847. doi: 10.1038/nri3562. Epub 2013 Nov 5. Nat Rev Immunol. 2013. PMID: 24189577 No abstract available.
-
Influenza vaccines: mTOR inhibition surprisingly leads to protection.Nat Immunol. 2013 Dec;14(12):1205-7. doi: 10.1038/ni.2764. Nat Immunol. 2013. PMID: 24240151 No abstract available.
References
-
- Pica N, Palese P. Toward a universal influenza virus vaccine: prospects and challenges. Annu Rev Med. 2013;64:189–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

