Resveratrol enhances ionizing radiation-induced premature senescence in lung cancer cells
- PMID: 24141489
- PMCID: PMC3834547
- DOI: 10.3892/ijo.2013.2141
Resveratrol enhances ionizing radiation-induced premature senescence in lung cancer cells
Abstract
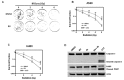
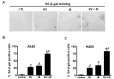
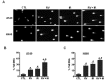
Radiotherapy is used in >50% of patients during the course of cancer treatment both as a curative modality and for palliation. However, radioresistance is a major obstacle to the success of radiation therapy and contributes significantly to tumor recurrence and treatment failure, highlighting the need for the development of novel radiosensitizers that can be used to overcome tumor radioresistance and, thus, improve the efficacy of radiotherapy. Previous studies indicated that resveratrol (RV) may sensitize tumor cells to chemotherapy and ionizing radiation (IR). However, the mechanisms by which RV increases the radiation sensitivity of cancer cells have not been well characterized. Here, we show that RV treatment enhances IR-induced cell killing in non-small cell lung cancer (NSCLC) cells through an apoptosis-independent mechanism. Further studies revealed that the percentage of senescence-associated β-galactosidase (SA-β-gal)-positive senescent cells was markedly higher in cells treated with IR in combination with RV compared with cells treated either with IR or RV alone, suggesting that RV treatment enhances IR-induced premature senescence in lung cancer cells. Comet assays demonstrate that RV and IR combined treatment causes more DNA double-strand breaks (DSBs) than IR or RV treatment alone. DCF-DA staining and flow cytometric analyses demonstrate that RV and IR combined treatment leads to a significant increase in ROS production in irradiated NSCLC cells. Furthermore, our investigation show that inhibition of ROS production by N-acetyl-cysteine attenuates RV-induced radiosensitization in lung cancer cells. Collectively, these results demonstrate that RV-induced radiosensitization is associated with significant increase of ROS production, DNA-DSBs and senescence induction in irradiated NSCLC cells, suggesting that RV treatment may sensitize lung cancer cells to radiotherapy via enhancing IR-induced premature senescence.
Figures



Similar articles
-
Resveratrol induces premature senescence in lung cancer cells via ROS-mediated DNA damage.PLoS One. 2013;8(3):e60065. doi: 10.1371/journal.pone.0060065. Epub 2013 Mar 22. PLoS One. 2013. PMID: 23533664 Free PMC article.
-
Activation of p53 with Nutlin-3a radiosensitizes lung cancer cells via enhancing radiation-induced premature senescence.Lung Cancer. 2013 Aug;81(2):167-73. doi: 10.1016/j.lungcan.2013.04.017. Epub 2013 May 16. Lung Cancer. 2013. PMID: 23683497 Free PMC article.
-
Inhibition of PI3 kinases enhances the sensitivity of non-small cell lung cancer cells to ionizing radiation.Oncol Rep. 2010 Dec;24(6):1683-9. doi: 10.3892/or_00001034. Oncol Rep. 2010. PMID: 21042768
-
The Molecular and Cellular Strategies of Glioblastoma and Non-Small-Cell Lung Cancer Cells Conferring Radioresistance.Int J Mol Sci. 2022 Nov 5;23(21):13577. doi: 10.3390/ijms232113577. Int J Mol Sci. 2022. PMID: 36362359 Free PMC article. Review.
-
Natural Radiosensitizers in Radiotherapy: Cancer Treatment by Combining Ionizing Radiation with Resveratrol.Int J Mol Sci. 2022 Sep 13;23(18):10627. doi: 10.3390/ijms231810627. Int J Mol Sci. 2022. PMID: 36142554 Free PMC article. Review.
Cited by
-
Natural Polyphenols Targeting Senescence: A Novel Prevention and Therapy Strategy for Cancer.Int J Mol Sci. 2020 Jan 20;21(2):684. doi: 10.3390/ijms21020684. Int J Mol Sci. 2020. PMID: 31968672 Free PMC article. Review.
-
Catalase inhibits ionizing radiation-induced apoptosis in hematopoietic stem and progenitor cells.Stem Cells Dev. 2015 Jun 1;24(11):1342-51. doi: 10.1089/scd.2014.0402. Epub 2015 Mar 3. Stem Cells Dev. 2015. PMID: 25603016 Free PMC article.
-
The Roles of Autophagy and Senescence in the Tumor Cell Response to Radiation.Radiat Res. 2020 Aug 1;194(2):103-115. doi: 10.1667/RADE-20-00009. Radiat Res. 2020. PMID: 32845995 Free PMC article. Review.
-
Comparison of the Effects of Resveratrol and Its Derivatives on the Radiation Response of MCF-7 Breast Cancer Cells.Int J Mol Sci. 2021 Sep 1;22(17):9511. doi: 10.3390/ijms22179511. Int J Mol Sci. 2021. PMID: 34502426 Free PMC article.
-
Therapeutic Implications for Overcoming Radiation Resistance in Cancer Therapy.Int J Mol Sci. 2015 Nov 10;16(11):26880-913. doi: 10.3390/ijms161125991. Int J Mol Sci. 2015. PMID: 26569225 Free PMC article. Review.
References
-
- Hall EJ, Wu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56:83–88. - PubMed
-
- Sale S, Tunstall RG, Ruparelia KC, et al. Comparison of the effects of the chemopreventive agent resveratrol and its synthetic analog trans 3,4,5,4′-tetramethoxystilbene (DMU-212) on adenoma development in the Apc(Min+) mouse and cyclooxygenase-2 in human-derived colon cancer cells. Int J Cancer. 2005;115:194–201. - PubMed
-
- Gullett NP, Ruhul Amin AR, Bayraktar S, et al. Cancer prevention with natural compounds. Semin Oncol. 2010;37:258–281. - PubMed
-
- Bhat KP, Lantvit D, Christov K, et al. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61:7456–7463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

