p34 is a novel regulator of the oncogenic behavior of NEDD4-1 and PTEN
- PMID: 24141722
- PMCID: PMC3857621
- DOI: 10.1038/cdd.2013.141
p34 is a novel regulator of the oncogenic behavior of NEDD4-1 and PTEN
Abstract
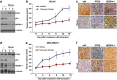
PTEN is one of the most frequently mutated or deleted tumor suppressors in human cancers. NEDD4-1 was recently identified as the E3 ubiquitin ligase for PTEN; however, a number of important questions remain regarding the role of ubiquitination in regulating PTEN function and the mechanisms by which PTEN ubiquitination is regulated. In the present study, we demonstrated that p34, which was identified as a binding partner of NEDD4-1, controls PTEN ubiquitination by regulating NEDD4-1 protein stability. p34 interacts with the WW1 domain of NEDD4-1, an interaction that enhances NEDD4-1 stability. Expression of p34 promotes PTEN poly-ubiquitination, leading to PTEN protein degradation, whereas p34 knockdown results in PTEN mono-ubiquitination. Notably, an inverse correlation between PTEN and p34/NEDD4-1 levels was confirmed in tumor samples from colon cancer patients. Thus, p34 acts as a key regulator of the oncogenic behavior of NEDD4-1 and PTEN.
Figures






References
-
- Tamura M, Gu J, Tran H, Yamada KM. PTEN gene and integrin signaling in cancer. J Natl Cancer Inst. 1999;91:1820–1828. - PubMed
-
- Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379–1386. - PubMed
-
- Tamguney T, Stokoe D. New insights into PTEN. J Cell Sci. 2007;120 (Pt 23:4071–4079. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

