FOXM1 targets NBS1 to regulate DNA damage-induced senescence and epirubicin resistance
- PMID: 24141789
- PMCID: PMC3969838
- DOI: 10.1038/onc.2013.457
FOXM1 targets NBS1 to regulate DNA damage-induced senescence and epirubicin resistance
Abstract
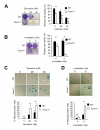
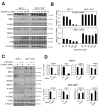
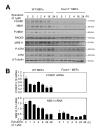
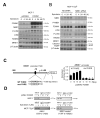
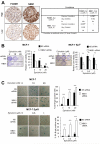
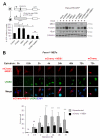
FOXM1 is implicated in genotoxic drug resistance but its mechanism of action remains elusive. We show here that FOXM1-depletion can sensitize breast cancer cells and mouse embryonic fibroblasts (MEFs) into entering epirubicin-induced senescence, with the loss of long-term cell proliferation ability, the accumulation of γH2AX foci, and the induction of senescence-associated β-galactosidase activity and cell morphology. Conversely, reconstitution of FOXM1 in FOXM1-deficient MEFs alleviates the accumulation of senescence-associated γH2AX foci. We also demonstrate that FOXM1 regulates NBS1 at the transcriptional level through an forkhead response element on its promoter. Like FOXM1, NBS1 is overexpressed in the epirubicin-resistant MCF-7Epi(R) cells and its expression level is low but inducible by epirubicin in MCF-7 cells. Consistently, overexpression of FOXM1 augmented and FOXM1 depletion reduced NBS1 expression and epirubicin-induced ataxia-telangiectasia mutated (ATM)phosphorylation in breast cancer cells. Together these findings suggest that FOXM1 increases NBS1 expression and ATM phosphorylation, possibly through increasing the levels of the MRN(MRE11/RAD50/NBS1) complex. Consistent with this idea, the loss of P-ATM induction by epirubicin in the NBS1-deficient NBS1-LBI fibroblasts can be rescued by NBS1 reconstitution. Resembling FOXM1, NBS1 depletion also rendered MCF-7 and MCF-7Epi(R) cells more sensitive to epirubicin-induced cellular senescence. In agreement, the DNA repair-defective and senescence phenotypes in FOXM1-deficent cells can be effectively rescued by overexpression of NBS1. Moreover, overexpression of NBS1 and FOXM1 similarly enhanced and their depletion downregulated homologous recombination (HR) DNA repair activity. Crucially, overexpression of FOXM1 failed to augment HR activity in the background of NBS1 depletion, demonstrating that NBS1 is indispensable for the HR function of FOXM1. The physiological relevance of the regulation of NBS1 expression by FOXM1 is further underscored by the strong and significant correlation between nuclear FOXM1 and total NBS1 expression in breast cancer patient samples, further suggesting that NBS1 as a key FOXM1 target gene involved in DNA damage response, genotoxic drug resistance and DNA damage-induced senescence.
Figures


References
-
- Stearns V, Davidson NE, Flockhart DA. Pharmacogenetics in the treatment of breast cancer. Pharmacogenomics J. 2004;4(3):143–53. - PubMed
-
- Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair. 2007 Jul 1;6(7):923–35. - PubMed
-
- Riches LC, Lynch AM, Gooderham NJ. Early events in the mammalian response to DNA double-strand breaks. Mutagenesis. 2008 Sep;23(5):331–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

