A small RNA activates CFA synthase by isoform-specific mRNA stabilization
- PMID: 24141880
- PMCID: PMC3831309
- DOI: 10.1038/emboj.2013.222
A small RNA activates CFA synthase by isoform-specific mRNA stabilization
Abstract
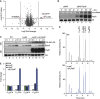
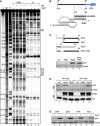
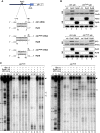
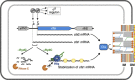
Small RNAs use a diversity of well-characterized mechanisms to repress mRNAs, but how they activate gene expression at the mRNA level remains not well understood. The predominant activation mechanism of Hfq-associated small RNAs has been translational control whereby base pairing with the target prevents the formation of an intrinsic inhibitory structure in the mRNA and promotes translation initiation. Here, we report a translation-independent mechanism whereby the small RNA RydC selectively activates the longer of two isoforms of cfa mRNA (encoding cyclopropane fatty acid synthase) in Salmonella enterica. Target activation is achieved through seed pairing of the pseudoknot-exposed, conserved 5' end of RydC to an upstream region of the cfa mRNA. The seed pairing stabilizes the messenger, likely by interfering directly with RNase E-mediated decay in the 5' untranslated region. Intriguingly, this mechanism is generic such that the activation is equally achieved by seed pairing of unrelated small RNAs, suggesting that this mechanism may be utilized in the design of RNA-controlled synthetic circuits. Physiologically, RydC is the first small RNA known to regulate membrane stability.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Antal M, Bordeau V, Douchin V, Felden B (2005) A small bacterial RNA regulates a putative ABC transporter. J Biol Chem 280: 7901–7908 - PubMed
-
- Argaman L, Hershberg R, Vogel J, Bejerano G, Wagner EG, Margalit H, Altuvia S (2001) Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr Biol 11: 941–950 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

