Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing
- PMID: 24142049
- PMCID: PMC5710001
- DOI: 10.1038/nbt.2696
Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing
Abstract
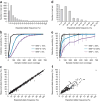
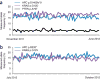
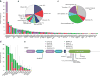
As more clinically relevant cancer genes are identified, comprehensive diagnostic approaches are needed to match patients to therapies, raising the challenge of optimization and analytical validation of assays that interrogate millions of bases of cancer genomes altered by multiple mechanisms. Here we describe a test based on massively parallel DNA sequencing to characterize base substitutions, short insertions and deletions (indels), copy number alterations and selected fusions across 287 cancer-related genes from routine formalin-fixed and paraffin-embedded (FFPE) clinical specimens. We implemented a practical validation strategy with reference samples of pooled cell lines that model key determinants of accuracy, including mutant allele frequency, indel length and amplitude of copy change. Test sensitivity achieved was 95-99% across alteration types, with high specificity (positive predictive value >99%). We confirmed accuracy using 249 FFPE cancer specimens characterized by established assays. Application of the test to 2,221 clinical cases revealed clinically actionable alterations in 76% of tumors, three times the number of actionable alterations detected by current diagnostic tests.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details are available in the online version of the paper.
Figures



Comment in
-
Next-generation sequencing in the clinic.Nat Biotechnol. 2013 Nov;31(11):990-2. doi: 10.1038/nbt.2743. Nat Biotechnol. 2013. PMID: 24213773 No abstract available.
References
-
- Stegmeier F, Warmuth M, Sellers WR, Dorsch M. Targeted cancer therapies in the twenty-first century: lessons from imatinib. Clin Pharmacol Ther. 2010;87:543–552. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

